Order: Piciformes
Family: Picidae
Genus: Colaptes
Species: Colaptes auratus
Subspecies: Colaptes auratus cafer
Colaptes (ko-LAP-teez)
Latinized from Greek, kolapto, to chisel or peck
auratus (aw-RA-tus)
Aurata, golden or gilded, from aurum, gold
cafer (KAY-fer)
After South Africa, a mistake made in 1788 by Johann Gmelin who believed they originally inhabited the area of the Xhosa, “Kaffir” people
(Lederer and Burr 2014)
Introduction
The Northern Flicker is a large woodpecker -12.5” L, 20” WS, 4.6 oz- with two distinct subspecies, including the Red-Shafted (Colaptes auratus cafer) and Yellow-Shafted (Colaptes auratus auratus) (Sibley 2003). While the Red-Shafted Northern Flicker is found in western North America and the Yellow-Shafted Northern Flicker is found in the east, they maintain a stable hybrid zone in the middle of the United States (Moore and Buchanon 1985). The western Red-Shafted subspecies of the Northern Flicker, as seen on the Evergreen campus and throughout Olympia, is identifiable from the bright red-orange rachis of the bird’s flight and tail feathers that stand out against its predominantly light brown-grey body. With black barring on the back and black spots on the belly, its black crescent-shaped breast band and white rump are also very distinctive features. A slight sexual dimorphism is exhibited by red-orange malar stripes that are characteristic of males, but lacking on the plain gray-brown head of females (Sibley 2003). Northern Flickers play an important role in forest communities and are often considered a keystone species due to their excavated tree cavities that provide important nesting habitat for many secondary-use species (Martin and Eadie 1999).
The Northern Flicker is the most common woodpecker in North America (Flockhart and Wiebe 2008). While Red-Shafted subspecies range west from the Rocky Mountains to the Pacific coast, Yellow-Shafted populations range east from the Rockies to the Atlantic coast (Moore and Koenig 1986). Along the Rockies and through the western Great Plains, as far south as Texas and north to Alaska, the well documented hybrid zone of Red-Shafted and Yellow-Shafted Northern Flickers is around 185-310 miles wide and 2,500 miles in length (AOU 1983 in Flockhart and Wiebe 2008). With such a wide geographic area, and in concordance with the ‘bounded hybrid superiority’ model of non-isolative reproductive stability, this hybrid zone is thought to be an ecotone for the species (Moore and Koenig 1986). Northernmost Red-Shafted Northern Flickers are migrants who breed in northern Canada and Alaska and winter further south in the United States, while those in California and the Rocky Mountains are known to partake in altitudinal migration, with some movement from higher elevations to lower elevations during the winter (Wiebe and Moore 2008).

McLane Creek Nature Trail, Olympia, WA
Photo: Savannah Smith
Found year-round across the continent, the wide distribution of Northern Flickers reflects their ability to survive in a variety of habitats. They are often observed in open woodlands and forest edges, but are also found in riparian areas, swamps, and burn areas (Wiebe and Moore 2008). They are also increasingly found in areas inhabited by humans and may be seen foraging in backyards or parks and heard drumming on artificial surfaces (Wiebe and Moore 2008). Northern Flickers are cavity nesters that excavate their cavities and nest in healthy, dying, or dead trees such as Douglas-fir (Psuedotsuga menziesii), lodgepole pine (Pinus contorta), and quaking aspen (Populus tremuloides) (Wiebe 2001). Unlike other woodpeckers, those in the genus Colaptes, such as our local Flicker, are often observed foraging on the ground in areas of short grass, bare soil, and anthills (Elchuck and Wiebe 2002).
As insectivorous woodpeckers with a preference for ants, Flickers often hop around and scratch at the ground to move leaves and expose ant nests (Bent 1939). They will repeatedly use their strong bill to probe into soil and extend their long tongues to capture ants and larvae found in the soil and under rocks and lichen (Wiebe and Moore 2008). With 45 to 99 per cent of their diet consisting of ants, they eat more of them than any other bird (Bent 1939, Gow, Wiebe and Higgins 2013), including genera such as Aphaenogaster, Camponotus, Cremastogaster, Formica, Lasius, Messor, Myrmica, Pheidole, Phrenolepis, Solenopsis, Tapinoma, and Tetramorium (Elchuck and Wiebe 2002). They commonly select foraging sites where ants are abundant and trees are nearby to offer protection. Wiebe and Gow have also found that Flickers maximize foraging efficiency by tracking the density of ants on the ground surface which results in a shift from foraging in the open when it was cold to foraging in shaded habitats when it was hot (2013). However, Northern Flickers do not forage exclusively in the ground. They may also be observed hopping along tree branches and moving both vertically on and around tree trunks, where they “hammer” at the trunks to search for and access prey (Peterson 2010). Flickers will also eat beetles, wasps, grasshoppers, crickets, caterpillars, and have been observed eating dogwood berries, huckleberries, serviceberries, elderberries, blackberries, and cherries during the fall and winter months when insects are less abundant (Bent 1939). Because Flickers are often in open fields of short grass or very little vegetation, they must be very alert and cautious in order to avoid avian and mammalian predators. Their cryptic back plumage is believed to reduce the risk of predation while foraging (Elchuck and Wiebe 2002). To maximize survival and access food sources, young Flickers have been found to move among habitat types and use areas with different vegetative characteristics compared to nest sites (Gow and Wiebe 2014). Like other woodpeckers, Flickers use their distinct zygodactyl feet (Proctor and Lynch 1993) – two toes in the front, two in the rear– in conjunction with their stiff tail, which is utilized like a third leg to brace on tree trunks.

Ant hill (genus Formica) – Common food source of the Northern Flicker
Supplier: Flickr: EOL Images Photographer: Hermann Falkner.
While the possible functional significance of the brightly colored rachis is unknown (Noble 1936), it is scientifically understood that carotenoid pigments synthesized by plants produce the bright pigments that occur in body plumage, and infrequently in flight feathers (Proctor and Lynch 1993). Analyses of fruits and seeds commonly eaten by Flickers have been found to contain such carotenoids as carotene and xanthophyll (Test 1969). Though carotenoids are rarely seen in flight feathers, the beautifully distinguishable wings of Flickers reveal an outrageous exception! While most of the red pigments in Red-Shafted Flickers are derived by the oxidization of carotenoids, it is possible that their consistently heavy diet of ants provides the red pigment that is deposited in their flight feathers, although carotenoid levels in adult ants have not been studied (Test 1969). During molt, Flickers can also use stored carotenoids in their body fat and liver for pigmentation (Test 1969).
Like many woodpeckers, Northern Flickers actively assert territoriality within the area immediately surrounding their nests (Elchuk 2003). Male Northern Flickers tend to play an equal to greater parental role (Gow and Wiebe 2012) to incubate the eggs at night, excavate the nest cavity, and actively defend breeding territories (Wiebe and Elchuk 2003a). Because males invest more time and energy into excavating cavities, incubating eggs, and caring for young, male survival rates are equivalent to those of females (Fisher and Wiebe 2006). Interestingly, this contrasts the survival rates of other bird species, where males tend to have higher survival rates (Fisher and Wiebe 2006).

N. Flicker using its stiff tail to brace against a tree. Source: Birds of the Northwest Playing Cards Artist: Stan Tekiela
Flicker clutch sizes range from 3 to 12 eggs (Gill 2007) and females will continue to lay eggs to replace those that disappear from the nest during the early incubation stage; a behavior exhibited in species distinguished as “indeterminate layers” (Bent 1939). In contrast to species recognized as indeterminate layers, species that are “determinate layers” lay a fixed number of eggs per clutch regardless of removal (Gill 2007). Like other woodpeckers, the eggs of Northern Flickers are very small in comparison to their body size, are relatively cheap to produce, and do not require a high energy investment from females (Wiebe 2006). The average 3 to 12 clutch range is exhibited by a latitudinal increase in clutch size (Koenig 1984). This pattern in clutch size, with an average increase of 1 egg per 10 degrees of latitude (Gill 2007), supports Ashmole’s seasonality hypothesis that suggests a direct relationship between geographic variation in clutch size and [seasonal] resource abundance (Koenig 1984). Although clutch size is linked to temperature, hatchling and fledgling success does not seem to be affected by temperature (Wiebe 2001). Incubation lasts from 14 to 16 days (Bent 1939) and as altricial young (Koenig 1984), hatchlings are helpless, featherless, and blind (Gill 2007).
Like other woodpeckers, Northern Flickers have a “flap-bounding” intermittent flight pattern that alternates between wing flapping and non-flapping, where the bird’s wings are folded against its body (Rayner, et al 2001)). This results in a flight pattern in which the bird may seem to “undulate” up and down along as it travels along its path of flight (Peterson 2010).
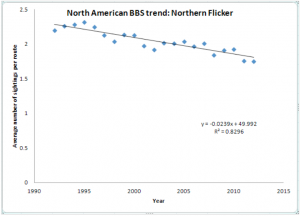
Pardieck, K.L., D.J. Ziolkowski Jr., M.-A.R. Hudson. 2014. North American Breeding Bird Survey Dataset 1966 – 2013, version 2013.0. U.S. Geological Survey, Patuxent Wildlife Research Center. Graph by Justin Averre.
Although Northern Flickers suffer from human induced habitat destruction, they have adapted well to disturbance and are often found in urban and suburban habitats. Although they are common residents of woodland habitats and are increasingly found around human habitation, Northern Breeding Bird Survey data indicate an overall decline in numbers of Northern Flickers. Though more research is needed to understand the true causes for population declines of Northern Flickers (Wiebe and Moore 2008), several hypotheses attempt to explain the causes for declines in Flicker population. Competition with European Starlings for nest cavities decreases available nesting habitat for Northern Flickers, thus affecting reproductive success (Ingold 1996). A study in 1994 found that Flickers lost their nest cavities to European Starlings both before egg laying and during the incubation period (Ingold 1996), and in 1998 the presence of nest boxes near active nest cavities had no effect to alleviate nest-site competitions with Starlings (Ingold 1998).
Other hypotheses describing the cause for decline in Northern Flicker populations include certain development and logging practices and the application of chemicals and pesticides on lawns, farmland, and fields- all of which can destroy suitable habitat for the birds (Wiebe and Moore 2008). Exurban development, or the development of parcels between 1 and 20 acres outside of municipal service areas, known as “ranchettes”, is a popular housing trend in the western United States (Merenlander et al 2009). Because they are less abundant in landscapes fragmented with ranchettes than undeveloped sites of the same habitat suite,Flickers are considered sensitive to development (Merenlender et al. 2009).

The N. Flicker and E. Starling have a highly competitive relationship
Supplier: Slater Museum of Natural History Photographer: Dennis Paulson
Postfire or salvage logging is the removal of dead, dying, or weakened trees, occasionally occurring with contention on public lands after fire or tree disease outbreak (Saab et al. 2011). Wildfire and bark beetles (Dendroctonus pondorosae) have increasingly affected great amounts of forest land over the past two decades, thereby increasing the amount of forest lands likely to be treated with salvage logging (McIver and Starr 2001, Beschta et al. 2004, Stephens and Ruth 2005, Lindenmeyer et al 2008 in Saab et al. 2011). Salvage logging removes habitat for cavity-nesting birds such as the Northern Flicker, and although survival rates for nests do not differ between the two, nest densities are significantly higher in unlogged compared to logged postfire forests (Saab et al. 2011).
A study on the survival rate of Flickers suggests that conservation efforts should focus on improving reproductive success in the species and survival of young instead of a focus on adult flickers (Fisher and Wiebe 2006b). Effective conservation will require continued research on the Northern Flicker to fully understand its unique habitat preferences and requirements.
LewisS_NOFL_call_11102012_932_47-0679_122-9704
Recorded by Stephanie Lewis, 10 November 2012, Olympia, Washington
LewisS_NOFL_call_11242012_1135_47-0802_122-9606
Recorded by Stephanie Lewis, 24 November 2012, Olympia, Washington
LewisS_NOFL_call_11102012_928_47-0679_122-9704
Recorded by Stephanie Lewis, 10 November 2012, Olympia, Washington
More audio resources are available at: The Macauly Library and xeno-canto
Blanc, L. A., and J. R. Walters. 2008. Cavity Excavation and Enlargement as Mechanisms for Indirect Interactions in an Avian Community. Ecology 89: 506-514.
Bower, A. R., and D. J. Ingold. 2004. Intraspecific Brood Parasitism in the Northern Flicker. The Wilson Bulletin 116: 94-97.
Brawn, J. D., S. K. Robinson and F. R. Thompson III. 2001. The Role of Disturbance in the Ecology and Conservation of Birds. Annual Review of Ecology and Systematics 32: 251-276.
Brigham, R. M., M. J. Sarell, and C. G. Harris. 1990. Roosting of Northern Flickers (Colaptes auratus) under a Concrete Bridge. Northwestern Naturalist 71: 52-53.
Dobkin, D S, A. C. Rich, J. A. Pretare, and W. H. Pyle. 1995. Nest-Site Relationships among Cavity-Nesting Birds of Riparian and Snowpocket Aspen Woodlands in the Northwestern Great Basin. The Condor 97: 694-707.
Edworthy, A. B., K. L. Wiebe, and K. Martin. 2012. Survival analysis of a critical resource for cavity-nesting communities: patterns of tree cavity longevity. Ecological Applications 22:1733–1742.
Elchuk, C. L. and K. L. Wiebe 2002. Food and predation risk as factors related to foraging locations of Northern Flickers. Wilson Bulletin 114: 349-357.
Elchuk, C. L. and K. L. Wiebe. 2003a. Home-range of Northern Flickers (Colaptes auratus) in relation to habitat and parental attributes. Canadian Journal of Zoology 81: 954-961.
Elchuk, C. L. and K. L. Wiebe. 2003b. Ephemeral food resources and high conspecific densities as factors explaining lack of feeding territories in Northern Flickers (Colaptes auratus). Auk 120: 187-193.
Fisher, R. J. and K. L. Wiebe. 2006a. Investment in nest defense by Northern Flickers: effects of age and sex. Wilson Journal of Ornithology 118: 452-460.
Fisher, R. J. and K. L. Wiebe. 2006b. Effects of sex and age on survival of Northern Flickers: a six-year field study. Condor 108: 193-200.
Fisher, R. J., and K. L. Wiebe. 2006. Nest Site Attributes and Temporal Patterns of Northern Flicker Nest Loss: Effects of Predation and Competition. Oecologia 147: 744-753.
Flockhart D. T. and A. L. Wiebe. 2008. Variable weather patterns affect annual survival of Northern Flickers more than phenotype in the hybrid zone. Condor 110(4):701-708.
Gentry, D. J., and K. T. Vierling. 2008. Reuse of Woodpecker Cavities in the Breeding and Non-Breeding Seasons in Old Burn Habitats in the Black Hills, South Dakota. American Midland Naturalist 160: 413-429.
Gill, F. B. 2007. Ornithology, Third Edition.W. H. Freeman and Company, New York.
Gow, E. A., and K. L. Wiebe. 2014. Survival and Habitat Use by Fledgling Northern Flickers in a Fragmented Landscape. The Journal of Wildlife Management 78: 273-281.
Gow, E. A., A. B. Musgrove, and K. L. Wiebe. 2013. Brood age and size influence sex-specific parental provisioning patterns in a sex-role reversed species. Journal of Ornithology 154: 525-535.
Gow, E. A., K. L. Wiebe,and R. J. Higgins. 2013. Lack of diet segregation during breeding by male and female Northern Flickers foraging on ants. Journal of Field Ornithology 84: 262-269.
Gow, E. A. and K. L. Wiebe. 2012. An unusually synchronous double brooding attempt by a Northern Flicker pair. Wilson Journal of Ornithology. 124: 389-392.
Ingold, D. J. 1996. Delayed nesting decreases reproductive success in Northern Flickers: implications for competition with European Starlings. Journal of Field Ornithology 67: 321-326.
Ingold, D. J. 1998. The Influence of Starlings on Flicker Reproduction When Both Naturally Excavated Cavities and Artificial Nest Boxes Are Available. The Wilson Bulletin 110: 218-225.
Koenig, W. D. 1984. Geographic variation in clutch size in the Northern Flicker (Colaptes auratus): support for Ashmole’s hypothesis. Auk 101: 698-706.
Kozma, J. M., and A. J. Kroll. 2012. Woodpecker Nest Survival in Burned and Unburned Managed Ponderosa Pine Forests of the Northwestern United States. The Condor 114: 173-184.
Lederer, R., and C. Burr. 2014. Latin for Bird Lovers. Timber Press, Portland, United States.
Martin, K., and J.M. Eadie. 1999. Nest webs: a community-wide approach to the management and conservation of cavity nesting forest birds. Forest Ecology Management 115: 243-257.
Martin, K, K. E. H. Aitken, and K. L. Wiebe. 2004. Nest sites and nest webs for cavity-nesting communities in interior British Columbia, Canada: Nest characteristics and niche partitioning. Condor 106: 5-19.
Merenlender, A. M., S. E. Reed, and K. L. Heise. 2009. Exurban development influences woodland bird composition. Landscape and Urban Planning 92: 255-263.
Moore, W. S., and D. B. Buchanan. 1985. Stability of the Northern Flicker Hybrid Zone in Historical Times: Implications for Adaptive Speciation Theory. Evolution 39: 135-151.
Moore, W. S., and W. D. Koenig. 1986. Comparative Reproductive Success of Yellow-Shafted, Red-Shafted, and Hybrid Flickers across a Hybrid Zone. The Auk 103: 42-51.
Noble, G. K. 1936. Courtship and sexual selection of the flicker (Colaptes auratus luteus). Auk 53: 269-282.
Peterson, R. T. 2010. Peterson Field Guide to Birds of Western North America. Houghton Mifflin Harcourt, New York.
Proctor, N. S., and P. J. Lynch. 1993. Manual of Ornithology. Yale University Press, New Haven, USA.
Rayner, J. M. V., P. W. Viscardi, S. Ward, and J. R. Speakman. 2001. Aerodynamics and Energetics of Intermittent Flight in Birds. American Zoologist 41: 188-204.
Saab, Victoria A., Russell, Robin E., Rotella, Jay, and Dudley, Jonathon G. 2011. Modeling nest survival of cavity-nesting birds in relation to postfire salvage logging. The Journal of Wildlife Management 75: 794-804.
Sadoti, G., T. J. Rodhouse, and K. T. Vierling. 2010. Spatial Dependence in Northern Flicker Habitat–Reproduction Relationships: An Application of Dutilleul’s Modified t-test. The Condor 112: 363-368.
Sibley, D. A. 2003. Northern Flicker (Colaptes auratus). Page 264 in The Sibley Field Guide to Birds of Western North America. Alfred A. Knopf, New York.
Test, F. 1969. Relation of wing and tail color of the woodpeckers Colaptes auratus and C. cafer to their food. The Condor 71: 206-211.
Wiebe, K. L. 2014. Responses of cavity-nesting birds to fire: testing a general model with data from the Northern Flicker. Ecology 95: 2537–2547.
Wiebe, K. L., and E. A. Gow. 2013. Choice of foraging habitat by northern flickers reflects changes in availability of their ant prey linked to ambient temperature. Ecoscience 20: 122-130.
Wiebe, K. L. 2001. Microclimate of tree cavity nests: Is it important for reproductive success in Northern Flickers? Auk 118: 412-421.
Wiebe, K. L. 2002. First Reported Case of Classical Polyandry in a North American Woodpecker, the Northern Flicker. The Wilson Bulletin 114: 401-403.
Wiebe, K. L. 2006. Egg composition in Northern Flickers. Condor 108: 977-980.
Wiebe, K. L. and W. S. Moore. 2008. Northern Flicker (Colaptes auratus), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://0bna.birds.cornell.edu.cals.evergreen.edu/bna/species/166adoi:10.2173/bna.166











Leave a Reply