Sugars
We know sugar primarily as table sugar, or, more formally, as sucrose. It’s extracted from sugarcane or sugar beets and reduced down to a sweet white grain. In more scientific terms, sugar generally refers to the simplest members of a group of molecules called carbohydrates.
Literally “watered carbon”, Carbohydrates are so named because they are made from carbon together with the two atoms that make water, hydrogen and oxygen. Carbohydrates are a very important group of molecules to the cook and in fact to all life as we know it. As Harold McGee explains in On Food and Cooking, “Carbohydrates are produced by all plants and animals for the purpose of storing chemical energy, and by plants to make a supporting skeleton for its cells”(803). So yes, Carbohydrates are important. They are the energy our cells run on and they are the skeleton of plant bodies. For culinary use it is this first group of carbohydrates Mcgee describes, the ones used for storing energy, that we call sugars. The structural group can be molecules like cellulose, which is indeed a carbohydrate. However we don’t often refer to Cellulose as a sugar, possibly because we can’t break it down and digest it.
Of the chemical group sugars, there are a few that are very common in cooking. These can divided into three groups: monosaccharides, disaccharides, and polysaccharides. These groups go in ascending order of complexity. Mono-(one) unit sugars, Di-(two) unit sugars, and poly-(many) unit sugars.
The monosaccharides, are the basic building blocks of the other sugar groups. They are basically rings of carbon atoms with hydrogen and oxygen atoms attached. However, the seemingly small differences in the arrangement of these basic rings and their hydrogen and oxygen hangers on turn out to make big differences in these molecules and how they behave in both nature and cooking. The three most important culinary monosaccharides are Glucose, Fructose, and Galactose. All of these simple sugars may appear similar in their molecular structure, but they actually taste and act differently because of little differences in the arrangement of their atoms (“Monosaccharides” Encyclopedia Britannica 2016). Glucose is extremely common. It is the simple sugar that we run our cells on. Fructose is the sweetest simple sugar and occurs naturally in fruits. Galactose is found in milk, combined with Glucose to form the Disaccharide Lactose.

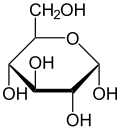

Fructose D-Glucose Galactose
www.wikipedia.org/wiki/Monosaccharide
When two of these monosaccharides get together, they become our second group, the disaccharides. These Disaccharides include the now ubiquitous cane and beet sugars – Sucrose (a combination of Fructose and Glucose). The natural sugar in milk, Lactose, is a disaccharide made from Glucose and Galactose (“lactose” Encyclopædia Britannica 2016).
Finally we have the polysaccharides. These are just long chains of monosaccharides. Sometimes they are simple strings of sugars other times they branch as intricately as a tree. Starch is a polysaccharide we’ve all cooked with. Glycogen, a form of sugar stored by animals in their meat, sometimes called “animal starch” (McGee, 2004, p. 804) is also a polysaccharide we’ve cooked, though probably without knowing it. We can’t taste sweetness directly from polysaccharides because they are such large molecules. However they do provide body that can fill our mouths, without necessarily stimulating our taste buds. Furthermore, our saliva has an enzyme called amylase that will actually break apart some polysaccharides as we chew them. Have you ever chewed on a cracker for 10 minutes? Biology teachers sometimes show this trick to their high school and middle school students. At some point in the interminable chewing the simple starchy cracker will become sweet. This transformation is actually the result of your mouth’s amylase enzyme breaking down the starch into mono- and disaccharides Glucose and Maltose, which we can taste. The sugars are there the whole time. They just need to broken down to a size that can interact with our taste buds.
Sugars are not only useful to cooks for their sweetness and energy, but for other cooking properties too. Sugars will thicken liquids, and tenderize breads. They will react with themselves to create caramel and they will react with proteins to start the Maillard reaction (see our Maillard page). The fact that there are sugars in all plants and animals tells us that we’re cooking with sugar even if we don’t add any cane or beet sugar to our food. So, it’s worth looking at what exactly the culinary sugars are and how they behave in cooking.
Again McGee proves to be an excellent source on this subject. He provides charts comparing the sweetness of the different sugars on a numerical scale compared to table-sugar (sucrose): sucrose at 100, fructose at 120, Glucose at 70, and Lactose down at 40 (McGee, p.655). The sugars differ in other ways too. Sucrose gives more body to liquids. It crystallizes at lower concentrations than Glucose or Fructose which tend to be found in syrup form such as honey, corn syrup, fruit juice concentrate. Sugar can be manipulated in many ways by sugar artists and pastry cooks, from caramels to cotton candy to sugar sculptures.
However, since the focus of this website is primarily on savory foods we will talk about the ways sugars react to produce caramelization and Maillard reactions.
Caramelization is essentially the breakdown and reaction of sugar molecules under heat. It produces brown color and a complex mix of flavors. McGee beautifully describes this transformation, writing, “From a single kind of molecule in the form of colorless, odorless, simply sweet crystals, the cook generates hundreds of new and different compounds, some of them small fragments that are sour or bitter, or intensely aromatic, others large aggregates with no flavor but a deep brown color.”(656) Infact McGee’s description, with a couple small changes, could apply to the other wonderful browning reaction that happens when we heat foods- the Maillard reaction. Because the Maillard reaction is a reaction of sugars with amino acids, the products of the Maillard reaction can include Sulfur and Nitrogen atoms from the amino acids in addition to the Carbon, Hydrogen and Oxygen atoms found in sugars. This ups the number of reaction products from hundreds (in the caramelization reaction) to thousands in the Maillard reaction (Golon and Kuhnert, 2014). As cooks we can understand simply that more reaction products equals more complex flavors. From a cook’s perspective, the Maillard reaction is usually desirable. It gives the savor to bread, the brown crust to a steak, and the roastiness to coffee.
So, since we are talking about sugars, how does the sugar side of the Maillard reaction work? It turns out that the part of the sugar molecules that react with with the Amino acids are the Carbonyl group (Helou et. al., 2016). The carbonyl group is a Carbon double bonded to an Oxygen. Sugars that have this group are called “Reducing Sugars” because they can play the reducing role in an oxidation reduction reaction, ‘giving’ their electrons to another atom or molecule. So, which sugars are reducing sugars? Which sugars can we expect to react in the Maillard reaction? All Monosaccharides are reducing sugars. However, the way sugars bond to each other to become Di- and Poly- saccharides often changes the carbonyl group. For example Sucrose is not a reducing sugar even though its two parts (Glucose and Fructose) would be reducing sugars on their own. Some di- and poly- can be reducing sugars (when they have a carbonyl group on the end of the chain of sugars) but, in general, the simple monosaccharides will be the most active group for starting Maillard reaction. Within the simple sugars group Fructose seems to be the most reactive. A study showed the different rates of Maillard reaction with different sugars, essentially by baking shortbread made with different kinds of sugars and quantifying how brown they got and the concentration of certain browning reaction products (Ameur, 2007). They found that at temperatures below 250 C (482 f) the cookies were, from most browned to least, Fructose, Glucose, and Sucrose. At higher temperatures the Sucrose started to produce lots of browning. Even though sucrose is not a reducing sugar, with enough heat it will break into its two Monosaccharide parts in a reaction called Hydrolysis (named this because it consumes a water molecule). Once broken apart the sucrose forming saccharides will readily undergo Maillard reaction.
With all this info, we thought we’d do our own experient. Since the Maillard reaction is sugar and protein reacting under heat we thought dulce de leche, a long slow cooked milk and sugar caramel, would be an excellent medium to work with. We made two batches of dulce de leche, Batch 1 with milk and cane sugar and Batch 2 with milk and a mix of half cane sugar half honey. We wanted to see if the different sugars in Honey would affect the rate of browning in the caramels. Even though the two dulce de leches were cooked right next to each other, with the same volume of sweeteners, they were completely different. Batch 2, with honey browned much faster than Batch 1 (with sucrose alone). It turns out that honey is mostly sweet from Fructose and Glucose because bees break up most of the sucrose in nectar as they transform it into honey (McGee, 665). We speculated that the Fructose in the honey was reacting with the amino acids in the milk faster than the Sucrose was breaking down and reacting in the other batch.
Far left: Sucrose and milk simmered for 45 minutes. Right: Sucrose, Honey and Milk simmered for 45 minutes (photo by Daniel Saunders)
Aside from the rate of browning we noticed a texture difference. Batch 1 (sucrose alone) was thicker than Batch 2. When they cooled this difference became even more noticeable as Batch 1 crystallized and looked like dry peanut butter. This is because Sucrose molecules are larger than Monosaccharides and obstruct water molecule movement more.
Above Left: cooled Dulce de leche from Batch 2 with honey Above Right: cooled crystallized dulce de leche from batch 1 with only cane sugar
Finally there was the flavor difference. Batch 1 had a nice sticky and fairly complex caramel and toasty flavor. Batch 2 had lost some of the subtle herbal and minty notes of the honey but it still had a sweet floral aroma that added pleasingly to the caramel and toasted and milky flavors.
When we made another batch of honey dulce de leche the next week we realized a problem. Honey has an organic acid called Gluconic acid which the bees make to help preserve the honey (McGee, 665). This lowers the pH to about 3.9 which seems to be enough to curdle milk if you heat milk with enough honey dissolved in it. You could end up with some kind of honeyed cheese curds instead of dulce de leche. We corrected this by adding a half teaspoon of baking soda to bring the milks pH closer to neutral. Many online dulce de leche recipes call for some baking soda anyway because it helps the amino acids engage in the Maillard reaction.
If you want to make our honey dulce de leche here’s the recipe:
Combine in a 4 quart saucepan,
-4 cups whole milk
-1/2 cup plus 2 Tbs cane sugar
-1/2 cup plus 2 Tbs honey
-1/4 tsp baking soda
Stir this mixture and heat gently until the sweeteners dissolve completely. Bring to a gentle boil, still stirring frequently. Turn down to the lowest setting and let the sweet milk cook. Keep stirring it once in a while. remember to scrape the bottom of the pot where the sugar can burn. It may take 50 to 90 minutes to reduce and caramelize. If you’er there to stir it, you can turn it up a little. Once its brown and thick and syrupy it’s done.
Works Cited
Ameur, Lamia Ait, Odile Mathieu, Valérie Lalanne, Gilles Trystram, and Ines irlouez-Aragon. 2007. “Comparison of the Effects of Sucrose and Hexose on Furfural Formation and Browning in Cookies Baked at Different Temperatures.” Food Chemistry 101 (4): 1407–16. doi:10.1016/j.foodchem.2006.03.049.
“Glucose | Biochemistry.” 2016. Encyclopedia Britannica. Accessed February 24. http://www.britannica.com/science/glucose.
Golon, Agnieszka, Christian Kropf, Inga Vockenroth, and Nikolai Kuhnert. 2014. “An Investigation of the Complexity of Maillard Reaction Product Profiles from the Thermal Reaction of Amino Acids with Sucrose Using High Resolution Mass Spectrometry.” Foods 3 (3): 461–75. doi:10.3390/foods3030461.
Helou, Cynthia, Philippe Jacolot, Céline Niquet-Léridon, Pascale Gadonna-Widehem, and Frédéric J. Tessier. “Maillard Reaction Products in Bread: A Novel Semi-quantitative Method for Evaluating Melanoidins in Bread.” Food Chemistry 190 (2016): 904-11. Web.
Kamal, Mohammad A., and Peter Klein. 2011. “Determination of Sugars in Honey by Liquid Chromatography.” Saudi Journal of Biological Sciences 18 (1): 17–21. doi:10.1016/j.sjbs.2010.09.003.
“lactose”. Encyclopædia Britannica. Encyclopædia Britannica Online.
Encyclopædia Britannica Inc., 2016. Web. 25 Feb. 2016
<http://www.britannica.com/science/lactose>.
McGee, Harold. 2004. On Food and Cooking. 2nd ed. Scribner.
“Monosaccharide | Chemical Compound.” 2016. Encyclopedia Britannica. Accessed February 25. http://www.britannica.com/science/monosaccharide.
Purlis, Emmanuel. 2010. “Browning Development in Bakery Products – A Review.” Journal of Food Engineering 99 (3): 239–49. doi:10.1016/j.jfoodeng.2010.03.008.






