
By Ltshears (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons
Family: Turdidae
Genus: Turdus
Species: Turdus migratorius
The American Robin (T. migratorius) has been called America’s best-loved bird. The robin is known as a welcome harbinger of spring’s arrival to the northern states, and its clear, liquid caroling greets early-risers before almost any other morning birdsong. The robin is above all adaptive, a dietary opportunist and colonizer of disturbed and edge habitats, whose proliferation across the North American continent was connected to the colonial spread of European culture in the western hemisphere. The robin’s red breast, its ubiquity, and its popularity make it one of the most recognizable birds of North America.

American Robin – Diet – Worms
By Brocken Inaglory (Own work) [GFDL or CC-BY-SA-3.0-2.5-2.0-1.0], via Wikimedia Commons
The robin’s most distinguishing feature is its red breast, described as ranging from “clear brick to rufous-red” (Wauer 1999). It has a white lower abdomen, and its back and wings are a dark brownish grey to black, with some populations exhibiting white tail corners. It has a blackish head, darker than its back and wings, with incomplete white eye rings, a yellow beak, and a white chin with black streaks. Males of the species tend to be darker overall, with more intense colors as compared to females, and juveniles sport distinct dark spots on their somewhat paler orange-to-red breast (Wauer 1999). Robins occasionally exhibit albinism, resulting in partial albinos with white feathers distributed throughout their plumage, White Robins, whose plumage is entirely white but who still retain some pigmentation, and total albinos, who completely lack pigmentation, and as a result have pink eyes (Eiserer 1976). Very rarely, robins exhibit melanism, which results in a very dark or entirely black plumage. Robins have, on average, 2900 feathers (Eiserer 1976).
The robin’s flight is described as very straight, with even wingstrokes. They fly at an average speed of 17-32 mph (Wauer 1999), with 3-4 wingflaps per second (Eiserer 1976).
Before the arrival of European colonists to the western hemisphere, the American Robin was called “Goshi” by the Dog-rib Indians of northwestern Canada, and “Cha La Cha Lee” by the northeastern Penobscots (Eiserer 1976). European settlers named it “robin” after their familiar and well-liked European Robin, which has a superficially similar bright orange breast, but is only very distantly related to the American Robin(Wauer 1999).
The westward migration of Europeans and their agricultural style and animals expanded the robin’s range immensely, with disturbed soil and earthworms imported into previously worm-free areas providing food, and grazing cattle clearing heavy underbrush in forested areas increasing availability of their preferred habitat (Eiserer 1976).
The robin is held in generally very high regard, and as one of the earliest-arriving springtime migrants is seen as a harbinger of spring. Up until the early 20th century, the robin was often hunted, eaten, and sold as food, especially in the Southern states, where there was less sentimental attachment to the species (Eiserer 1976).
The American Robin is the state bird of Michigan, Connecticut, and Wisconsin (Tait 2005), and was featured on Canada’s 2 dollar bill (Wauer 1999).
The American Robin featured prominently in Rachel Carson’s 1962 book Silent Spring, a treatise on the environmentally harmful effects of indiscriminate pesticide use, which contributed to the banning of the pesticide DDT in 1972 and signaled the beginning of a shift in public consciousness regarding environmental protection (Wauer 1999).
![By Ken Thomas [Public domain], via Wikimedia Commons](http://blogs.evergreen.edu/birds/files/2012/11/American_Robin-rangemap-300x225.gif)
By Ken Thomas [Public domain], via Wikimedia Commons
The yellow section shows the American Robin breeding range.
The green section shows the American Robin year round range.
The blue sections shows the American Robin non-breeding range.
The American Robin is a widespread species, ranging throughout North and Central America as far north as the arctic circle and as far south as Guatemala. Robins can be found from the East to the West Coast, from sea-level up to the treeline in mountainous areas. It’s range has expanded as a result of anthropogenic changes to the landscape which have increased available habitat (Eiserer 1976).
Habitat preference is closely tied to life history traits, especially dietary and nesting needs. In the absence of human settlement, the robin’s preferred habitat is in forest clearings with damp ground and short grass (Wauer 1999). Such habitats provide foraging for invertebrates, wild fruit-bearing trees, and suitable nesting sites (Wauer 1999). In human-impacted areas, the robin favors cleared and second-growth habitats (Gill 2007). Suburbs also fulfill its criteria nicely, with the exposed soil of gardens enabling highly productive foraging, and sturdy low-limbed trees for nesting (Eiserer 1976). In winter the robin prefers to stay in large flocks in wildlands further removed from human habitation (Wauer 1999).
Robins are dietary generalists and opportunists. While they have dietary preferences, they are also known to sometimes eat surprising foods. In the summer they feed mainly on the ground on insects and other invertebrates, and in the fall and winter mainly on fruits and berries growing on trees, shrubs, and vines, with their year round diet averaging 58% plant-based and 42% animal-based (Wauer 1999).
The popular image of the robin with an earthworm dangling from its beak notwithstanding, its preferred animal food is the caterpillar, followed by beetles, and then earthworms (Wauer 1999). Plant-food preferences vary locally, but T. migratorius is especially fond of members of the rose family, including cherries and plums (Wauer 1999). Color was found to play a significant role in fruit selection (Lepczyk et al. 20000). The findings of one study suggest that robins sometimes prefer invasive fruits to native ones, and that they are somewhat willing to test novel foods, even if familiar foods are present (LaFleur et al. 2007). Robins have been reported to occasionally eat a large range of animals, including fish, marine invertebrates, and snakes (Wauer 1999).
At the start of the breeding season, less than 1% of the robin’s diet is comprised of fruit, shifting to 90% by late summer (Wauer 1999). A laboratory experiment with artificial fruit and migratory American Robins found that robins prefer sugar-rich to lipid-rich fruits overall due to their greater ease of digestion and greater rate of energy gain (Lepczyk et al. 2000). However, robins undergo seasonal physiological changes that increase their ease of assimilation of lipids in tandem with seasonal changes in fruit availability, and, consequently, robins prefer to eat more prevalent lipid-rich autumnal fruit as the season progresses, enabling them to build fat reserves prior to migration (Lepczyk et al. 2000).
The life expectancy of an adult robin is ten years, but high nestling mortality brings average life expectancy down to one year and two months. The maximum recorded age for a robin in captivity is 17 years (Wauer 1999).
Spring Migration
Robin migration is “governed mainly by trends of temperature” (Eiserer 1976), with northward migration moving along the 2ºC isotherm- the gradient of the main spring thaw along which local temperatures are on average above freezing, which increases in latitude as spring progresses in the northern hemisphere (Gill 2007). Robins migrate in large flocks, sometimes flying both day and night (Wauer 1999), but generally preferring daytime migration, and avoiding crossing large bodies of water (Eiserer 1976). Males arrive at the breeding grounds before females and establish their territories, with older, more experienced males arriving first and claiming better sites (Wauer 1999). Many robins have high nest-site fidelity, with 55% returning to the area where they had been hatched the year before (Eiserer 1976) and 74% returning to within 10 miles of their birthplace (Wauer 1999). This site-fidelity contributes to breeding isolation of local populations, and may be a factor in sub-species divergence and eventual speciation. One study has shown that a bird does not return specifically to the area in which it hatched, but rather to its place of migratory departure, which in nature is almost always its place of birth (Eiserer 1976).
Territory
After large winter flocks arrive at breeding sites, they break down as males become more aggressive and territorial, restricting their movements to the area around their general territory, which may overlap with others and becomes fully established with the arrival of a female mate. At this point both members of the breeding pair defend the territory, whose borders remain somewhat fluid (Stokes 1979), with frequent trespassing (Eiserer 1976). Nesting territories range from 1/3-3/4 acres (Wauer 1999). The territory is used for mating, nesting and most of the pair’s feeding, but they may leave for some food, for nesting materials, and for the nightly communal roost. Robins roost together year-round (Stokes 1979), with males beginning upon their arrival on the breeding grounds, juveniles joining as they fledge, and females joining after their last brood of the season is fledged (Eiserer 1976). Robins share a communal feeding ground during the breeding season (Wauer 1999).
Mating
Robin courtship and display remain somewhat mysterious, with the main indication of courtship being the exclusive presence of two birds on a territory (Stokes 1979). Anecdotal evidence alternately suggests some level of male display or virtually no display at all (Wauer 1999). There is some suggestion that females engage in territory selection, selecting male mates based on the quality of their territory rather than display (Eiserer 1976).
One study has found evidence of assortative mating based on darkness and richness of breast color and damage to tail spots (Rowe and Weatherhead 2011). Mutual ornamentation may be the result of mutual sexual selection, or may be evidence of non-adaptive genetic correlations (Rowe and Weatherhead 2011). In the case of robins these ornamentations appear to be adaptive, with the same trait signaling different aspects of quality in each sex. In females these traits correlated with higher breeding performance, while in males darker breast plumage correlated to better body condition (Rowe and Weatherhead 2011).
Nesting
Nesting generally begins in mid-April (Wauer 1999), with nesting heights usually ranging between 5-30ft (Stokes 1979), and sometimes up to 60ft (Eiserer 1976). A site must provide firm foundation for the statant nest (Eiserer 1976), and the first nest of the season is often constructed in evergreen trees, with nests for subsequent broods more often situated in deciduous trees whose leaves have come in, providing cover (Wauer 1999). Edge habitats are preferred over deep forest, and nests are sometimes constructed on man-made structures (Eiserer 1976). In desert-riparian habitats, robins have been found to nest in habitats with relatively sparse vegetation, and may orient their nests based on latitude and altitude to maximize morning warmth from the sun and afternoon shade (Warkentin et al. 2003). One study in southern Quebec found that robins rarely nested on man-made structures, and enjoyed greater nesting and fledging success in suburban rather than urban areas, perhaps due to more feeding opportunities at sites with more bare soil and artificially shortened vegetation (Morneau et al. 1995). Duration of nesting activity was also found to be longer in suburban habitats, perhaps due to warmer, more stable temperatures and artificial illumination (Morneau et al. 1995).
Site selection and nest construction are undertaken primarily by the female, who may begin construction on more than one nest before settling on a site (Wauer 1999), and may reuse old nests, including nests of other species (Eiserer 1976). Construction takes from 5-6 days for the first nest of the season, and often less time for subsequent rests (Eiserer 1976). The male provides some materials for construction, which occurs in three distinct phases. First, a frame is constructed of sturdier twigs and grasses (Wauer 1999). Next, an inner cup is built up with mud, carried by the female in her beak and then shaped with breast and wings, making environmental moisture important for nest-building. Finally, an inner lining of finer, softer materials is added. Materials are adjusted for local conditions, with a greater use of lichen and moss at higher latitudes and altitudes (Eiserer 1976). Nests average 6-7in across the top and 3in high, with the inner cup 4in wide and 2.5in deep. The female often selects a new nesting site for each subsequent brood.
Robins may nest in close proximity to other species, and there have been observations of interspecies nesting (Wauer 1999). One study found a robin and a Northern Cardinal engaging in mixed-clutch nest sharing, alternating incubation shifts and sometimes both occupying the nest at the same time (Govoni et al. 2009). Only the robin young were hatched and fledged successfully. It may have been that the scarcity of quality nest sites resulted in this arrangement, or the cardinals may have been inexperienced juveniles (Govoni et al. 2009). At Malheur National Wildlife Refuge in Southeastern Oregon, the Eastern Kingbird has been observed refurbishing and re-using old American Robin nests more than those of any other bird (Cancellieri and Murphy 2013).
Laying and Incubation

By Laslovarga (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia CommonsEggs are usually laid in the early morning, with one egg laid per day, and with a typical total clutch size of 4 eggs, though clutches of up to 7 eggs have been observed(Wauer 1999). Eggs are bright blue, though sometimes greenish initially, and average 23.8-31.6mm long and 16.8-20.3mm wide.
Female robins most often choose between two incubation tactics. They either begin incubating before all eggs are laid, leading to staggered hatching times, or immediately upon completion of a clutch, which increases hatching synchrony (Rowe and Weatherhead 2009). One study found that robins sometimes use a third tactic, with approximately 20% of females delaying onset of incubation up to 4 days beyond clutch completion (Rowe and Weatherhead 2009). This increases hatching synchrony, but also increases nesting time, which incurs a higher cost in risk of predation (Rowe and Weatherhead 2009). The benefit of this tactic over incubating upon clutch completion is uncertain, as no significant differences were found in the number of young hatched or fledged. It may be that exerting control over when eggs hatch enables females to time hatching to coincide with food availability. Delayed-onset incubators tended to nest earlier, and the longer incubation was delayed, the longer eggs were incubated before hatching (Rowe and Weatherhead 2009). Average incubation time is 12-14 days (Stokes 1979), with females incubating almost exclusively with a 60-80% daytime attentiveness (Eiserer 1976). The male will sometimes provide the incubating female with food, but more often she leaves the nest to feed herself (Wauer 1999).
Hatching
Robin hatchlings are altricial, born blind and almost totally nude (Wauer 1999) at an average weight of 5 grams (Eiserer 1976). Upon hatching, parents carry the shell fragments away from the nest to avoid predators. As the nestlings have no capacity for thermoregulation, the female broods them for the first few days after hatching, and as necessary to protect them from heat or cold beyond that point (Eiserer 1976). The young birds excrete fecal sacs surrounded by gelatinous membrane which parents carry away from the nest or consume in order to maintain nest sanitation (Gill 2007).
Both parents contribute equally to feeding, initially by regurgitating partially digested food, and eventually transitioning to complete worms and larger insects (Eiserer 1976). As with adults, the main animal food source is caterpillars (Eiserer 1976). Nestlings beg by gaping their mouths, the bright orange color of which may stimulate a mechanical feeding reaction in the parent, who tend to feed the nestlings that beg the most (Eiserer 1976).The voracious nestlings consume an average of 3.2lbs per day per brood and develop rapidly (Wauer 1999), increasing their mass by 1000% in 10 days (Eiserer 1976). Their eyes open around day 6, and they are fully feathered by day 8 (Wauer 1999). Nestlings fledge on average 13 days after hatching, but may leave the nest earlier if disturbed (Eiserer 1976).
Fledging
After leaving the nest fledglings rarely return, and all parental care is provided by the male, allowing the female to begin a second brood (Wauer 1999). For a period of 2-4 weeks, the young birds can only flutter or fly weakly, and they follow the adult male around begging to be fed. During this time the male returns to the communal roost at night. Once the fledglings develop full flight capabilities and become self-sufficient foragers, they join the adult males at the evening roost, as do the female birds once their final brood of the season is fledged(Eiserer 1976). 40% of robin eggs laid lead to successfully fledged young (Eiserer 1976).
Molting
Robins undergo one molt per year between late July and early October, after their breeding season and before their fall migration (Wauer 1999). Young birds begin replacing their juvenile spotted contour feathers in midsummer, but do not replace their first wing feathers until their second molt (Gill 2007).
Fall Migration
During their fall migration, robins migrate generally south at a discontinuous, temperature-dependent rate of 20-30 miles per day, and tend to be relatively restricted longitudinally (Eiserer 1976). Not all robins from one breeding grounds migrate to the same wintering grounds (Eiserer 1976), and many do not return to the same wintering grounds in successive years (Wauer 1999). 80% of American Robins migrate to the gulf states (Eiserer 1976). Some robins migrate vertically, with breeding grounds at higher elevations than wintering grounds, but at the same latitude (Eiserer 1976). Migratory birds often form very large flocks on their wintering grounds, frequently comprised of up to 50,000 individuals (Eiserer 1976).
A very small proportion of robin populations are non-migratory (Eiserer 1976), wintering at their summer breeding grounds. As robins have been shown to be very tolerant of low temperatures (Eiserer 1976), temperature-driven migration may be based on an inability to derive enough sustenance from the environment to compensate for the increased metabolic demands of lower temperatures, and so these non-migratory populations may be exploiting more resource-abundant habitats, or may be more successful foragers (Eiserer 1976).
Predators and Competitors
The robin’s most formidable predator is the housecat. Domesticated cats and dogs are especially dangerous to fledglings, while some mammals and birds, especially corvids, prey on eggs and nestlings (Eiserer 1976). Robins’ tendency to fly out in open spaces makes them vulnerable to raptors, and they are often preyed upon by hawks and owls. Other birds, especially gulls, have been known to steal food from robins, and nest-site competitors include Mourning Doves and House Sparrows, which may evict robins (Wauer 1999). Human-related mortality results from vehicles and pesticide use, as well as intentional killing for crop protection and historically for food (Eiserer 1976).
Foraging
When feeding on the ground, robins search by moving about in short spurts punctuated by watchful immobility (Wauer 1999). They exhibit a distinctive head-cock behavior in which they lean down slightly and cock the side of their head at the ground as though listening intensely, often immediately before striking at prey. After a long-running argument as to the purpose of the head-cock, the conclusive verdict is that the robin does not track its prey auditorily, but in fact focuses one eye on the ground for a close visual inspection and visually-guided strike (Wauer 1999).
Giving-up density is the density of food in a patch being foraged at which an animal will move on to another area, as the value of the food, based on its rate of harvest, decreases to less than the cost in energy required for foraging (Oyugi and Brown 2003). Robins have lower giving-up densities the closer they are to cover from shrubs or canopy. They tend to forage more in the morning than in the afternoon, and tend to move to more open areas in the afternoon, possibly because they have depleted the safer foraging areas earlier in the day (Oyugi and Brown 2003).
Several studies have found that invertebrate and fruit foraging skills are learned by juvenile robins and improved over time (Vanderhoff and Eason 2007, 2008a, 2008b). Juveniles foraging for invertebrates have a lower proportion of strikes that end in capture, but compensate by making more strikes, at a greater energy cost, resulting in an equivalent rate of mean capture per minute (Vanderhoff and Eason 2007). Juveniles were observed to be less successful at picking cherries, but once having picked them were no more likely to drop a cherry than an adult (Vanderhoff and Eason 2007). Mulberries are the first fruit in the area of one study to ripen after juveniles become independent, and juveniles are significantly less successful at obtaining them, supporting the above findings. As a result of their less-developed foraging abilities, “starvation is major cause of juvenile mortality” (Vanderhoff and Eason 2008a).
Robins generally capture arthropods in sunny habitats and earthworms in moist soil, as worms and other invertebrates move close to the surface or above ground when soil is wet. Juveniles were found to be less successful at capturing arthropods, and less efficient at catching worms as compared to adults (Vanderhoff and Eason 2008b). Juveniles forage in groups with other robins more often than adults do, and may use the presence of other robins to distinguish good foraging habitat and suitable prey due to lack of experience and ability (Vanderhoff and Eason 2008b).
There are many reports that robins sometimes become intoxicated from the consumption of fermented fruit, causing a lack of coordination and sometimes leading to unconsciousness (Wauer 1999).
Flight Initiation Distance
One study found that robins can recognize the gaze direction of an approaching human and base their risk assessment on whether they are being watched. Robins showed longer flight initiation distances- the distance between an individual and an approaching threat when the individual flees- when they were watched directly while being approached (Eason et al. 2006). They were also warier of humans walking off of paths than on paths, and juvenile robins had longer flight initiation distances than adults (Eason et al. 2006). Another study found that robins tend to have shorter flight initiation distances in urban areas than in rural areas (Clucas and Marzluff 2012).
Territoriality
Robins are only territorial during their breeding season (Eiserer 1976), at which times they become more aggressive toward other robins the closer they are to their own nest. In territorial conflicts, the owner is most often the winner, and is more likely to be victorious the closer he or she is to the center of their territory (Wauer 1999). Female robins on average win more of their territorial battles than males, at 75% and 70% respectively (Wauer 1999).
Territorial posturing behaviors include (Stokes 1979):
“Attack-run”: rushing at an opponent with body low to the ground.
“Tail-lift”: facing an opponent with head lowered and tail lifted.
“Pushing”: making short movements toward an opponent, causing them to back up progressively. Also termed “supplanting” (Eiserer 1976).
“Wing-droop”: holding wingtips down below the level of the tail, before or after aggressive displays.
Robins sometimes help neighbors defend their nests against predators by mobbing, sending up an alarm call that draws many conspecifics to attack and harass the intruder by diving, striking, and snapping their beaks to make aggressive clicking sounds (Wauer 1999). Robins are more tolerant of close human presence during the day and during breeding season (Eiserer 1976).
Anti-Parasite Behavior
The American Robin is one of over 240 hosts of the Brown-headed Cowbird, Molothrus ater, an obligate brood parasite (Lang et al. 2014). Ejection of parasitic eggs is the most effective method of anti-parasite behavior. Others include nest vigilance, aggressive nest defense, and nest desertion. However, most Cowbird hosts accept parasitic eggs (Croston and Hauber 2013). Evolutionary equilibrium hypothesis holds that if the benefit of ejecting a brood parasite’s eggs, measured in saved energy and resources, is outweighed by the cost, measured in potential young lost to an individual if their egg ejection behavior is likely to damage their own eggs, then accepting brood parasites’ eggs may be adaptive (Rasmussen et al. 2009). Methods of nest ejection include puncture ejection, in which the parasite egg is punctured and carried using the hole created, and grasp-ejection, in which the entire unbroken egg is grasped in the beak and carried out (Rasmussen et al. 2009). Robins generally grasp-eject the parasitic eggs of the Brown-headed Cowbird with no damage to their own eggs (Rasmussen et al. 2009), and reject close to 100% of cowbird eggs (Lang et al. 2014).
In an experiment designed to determine whether variation in robins’ responses to cowbird brood parasitism was due to evolutionary genetic differences or phenotypic plasticity, researches placed dummy eggs in the nests of robins that had long bred outside the range of cowbirds (Kuehn et al. 2014). These birds were found to exhibit the same egg-rejection behaviors, though with a reduced level of responsiveness, as robins that breed in sympatry with cowbirds (Kuehn et al. 2014). Eggs were also placed in nests of robins breeding within the breeding range of cowbirds, but where cowbirds were almost completely absent. These robins, deemed “cowbird-naive”, were presumed to be closely related to nearby robin populations in areas where cowbirds were abundant, in whose nests dummy eggs were also placed. That the anti-parasite behaviors of these two populations were near identical shows that exposure to cowbirds is not a trigger for the expression of these behaviors (Kuehn et al. 2014).
That rejection behavior is reduced in allopatric populations long-isolated from parasites, but persists at equal levels in recently diverged populations in both the presence and absence of parasitism, rules out phenotypic plasticity as the source of the behaviors of the allopatric populations, and shows that the decline of rejection behavior is a genetic phenomenon (Kuehn et al. 2014). Persistence of this conditionally expressed behavioral trait in the absence of selective pressure indicates a low cost of maintenance of the trait. However, if the trait’s decline is a product of selective adaptation, there should be some cost for the maintenance of the trait in the absence of parasites, due for example to recognition errors leading to damage or rejection of ones’ own eggs (Kuehn et al. 2014). Evidence for such recognition errors is weak, and so a decline in rejection behavior is considered likely to be the result of genetic drift rather than selection against the trait(Kuehn et al. 2014).
One study found that robins distinguish parasitic eggs through criteria that include overall perceivable difference in color, with input from all four avian photoreceptors, but that cowbird eggs appear so similar in color to robin eggs when viewed with robins’ eyes that robins must be attuned specifically to cowbird eggs in a way that includes other parameters (Croston and Hauber 2013), likely to be a combination of size, spotting pattern, and background color (Lang et al. 2014). There is strong support for true egg recognition, that robins recognize their own eggs and compare them to parasitic eggs, over the discordancy hypothesis, which posits that they perceive the difference between eggs but do not recognize one or the other type as their own and eject the eggs with a minority of representation in the clutch (Lang et al. 2014).
Robins are more likely to eject cowbird eggs later in the breeding season, suggesting that the behavior may require a development period (Lang et al. 2014). Ejection is also more likely if there are more parasitic eggs added to the clutch, a defense against multiple parasitism, which significantly lowers a host’s fitness (Lang et al. 2014).
Hygiene
Bathing
Robins often bathe by standing in shallow water, dipping their body down and splashing around. They have been observed sun-bathing with wings spread (Wauer 1999).
Preening
As part of the maintenance of its plumage, the robin nibbles or draws its feathers through its bill, removing oils, dirt, and ectoparasites, as well as spreading fresh oil secreted by the uropygial gland at the base of its tail.(Wauer 1999)
Anting
Anting is the robin’s practice of picking up ants, crushing them, and rubbing their fluid on its feathers as though preening. Robins have been observed sitting or lying on anthills and allowing ants to swarm into their feathers. The purpose of this behavior is unknown, but possible explanations include feather maintenance (Wauer 1999), sensual pleasure, or that it repels parasites with the formic acid contained in ants’ bodily fluids. The practice may soothe skin irritation, having most often been observed during the robin’s molting period in late summer (Eiserer 1976).
Robin song is characterized as a “liquid”, “clear caroling”. It consists of 2-3 phrases alternated and repeated, with emphasis on particular phrases rotated through individual’s song repertoire. Phrases fall generally into two categories: carols and hisselys. Caroled phrases include the “zee-oo-zee” phrase, consisting of three buzzy notes with the middle a lower-pitched tone, and the “weep”, a distinct rising note. Hisselys are high-pitched thin whistles, often occurring as punctuation before a pause (Kroodsma 2005).
Robins sing most loudly at dawn and dusk (Wauer 1999), and generally prefer a minimum perch height of about 12ft for their performances (Eiserer 1976). Singing is strongly related to intensity of light (Eiserer 1976). Artificial illumination in areas inhabited by humans has been shown to result in robins beginning to sing earlier in the day, often starting their morning chorus during “true night”, which is almost never observed in areas devoid of artificial light (Miller 2006). These findings beg the question of whether the increased energetic demands of earlier singing in more brightly lit areas reduces fitness, or whether perhaps longer periods of illumination and alertness increase foraging productivity thanks to longer active foraging periods (Miller 2006).
Although most sources agree that only the male of the species sings (Kroodsma 2005, Stokes 1979), some maintain that the female does as well, albeit rarely (Wauer 1999). There is uncertainty as to the function of song in robins. In most birds there is a clear connection between song, territorial acquisition and defense, and mating, but in robins song does not appear closely tied to these functions, though they do sing most just prior to hatching in spring and early summer (Stokes 1979). One study found that robins invent or improvise most elements of their songs, but imitate some elements from their neighbors, and that there may be a predisposition to learn from relatives (Johnson 2006). Robin calls, on the other hand, are likely genetically programmed (Kroodsma 2005).
The robin’s alarm calls include the low, mellow “tut-tut”, a sharp explosive “piik”, and “quiquiquiqui”, a rapid series rising and falling in pitch (Kroodsma 2005). They also exhibit a distinctive thin, high pitched “seet” call, which anecdotal accounts have described as an aerial alarm (Vanderhoff and Eason 2009). One study confirms this, showing that robins only give the “seet” call to warn of aerial predators, which resulted in a greater duration of anti-predator behaviors, including skygazing, scanning, and alert state (Vanderhoff and Eason 2009). The aerial alarm was likely evolved to warn conspecifics, with two generally recognized explanations being reciprocal altruism and kin selection, though the relatedness of individuals in foraging flocks is unknown. Another possibility is that the “seet” call, due to its sonic qualities, confuses the predator, making the caller more difficult to locate and decreasing the likelihood of their being targeted, thereby increasing the caller’s direct fitness (Vanderhoff and Eason 2009).
Robins’ response to the “seet” aerial alarm call has been shown to depend on the intensity of the call as well as the age of the responder, suggesting that juveniles learn appropriate responses to the call over time. Alarm call intensity can indicate predator type and level of response. Robins’ responses include decreased foraging and increased anti-predator behaviors, including scanning, standing alert, and skygazing (Vanderhoff and Eason 2008c). That adults spend more time skygazing after a high intensity alert indicates that robins can recognize calls of varying intensity (Vanderhoff and Eason 2008c).
American Robin Call – Elisabeth Peelor
American Robin Call – Faron Watts – November 10 2012
Robin Call-tut-tut– Recorded by M. Szetela Nov. 2014 at Mimir’s Grove Farm
Robin Call-various-Recorded by M. Szetela Nov. 2014 at Mimir’s Grove Farm
Robin Call-piik– Recorded by M. Szetela Nov. 2014 at Mimir’s Grove Farm
Robin Call-quiquiqui-Recorded by M. Szetela Nov. 2014 at Mimir’s Grove Farm
Turdus migratorius is a species of Least Concern according to The IUCN Red List of Endangered Species. Due to the species large range size it doesn’t fall under the Vulnerable category and its population trend is currently increasing.
Global warming has impacted American Robins, The 2009 State of the Birds shows that robins are laying their eggs 14 days earlier than they did back in 1981 in their rocky mountain breeding grounds due to the climate change in North America (Sauer et al. 2001).
Thanks to the Christmas Bird Counts, the Audubon Society has determined that 58 percent of the 305 bird species have shifted North in the winter. The American Robin was one of those 305 species and has moved more than 200 miles over the past 40 years (Luning 2006).
Robins have been found to be a competent host for West Nile Virus (Kilpatrick et al. 2006), meaning that they have the ability to maintain the pathogen and transmit it to a feeding vector (Simpson et al. 2011). The mosquito Culex pipiens is one of the dominant vectors of WNV in the US. (Kilpatrick et al. 2006), and robins are its favorite host as determined by analysis of the mosquito’s blood meals to determine source species (Simpson et al. 2011). One study found that approximately 13% of robin blood meals detected in C. pipiens tested positive for West Nile Virus, with infections peaking in late August and early September (Lampman et al.2013). Robins respond differently to the range of WNV doses they receive from natural mosquito vectors. Higher doses correlate positively with the proportion of birds that become viremic, as well as with oral shedding of WNV RNA, which may be a risk to birds, predators, and humans that handle infected robins (VanDalen et al. 2013). However, American Robins do not experience high mortality due to West Nile Virus infection (Lampman et al.2013), regardless of dose (VanDalen et al. 2013). Additionally, one study found 18% of robins to be infected with trypanosomes, a hemoparasite that may interact with WNV and increase mosquito host competence (Hamer et al. 2013). Robin dispersal and migration in the fall drives a seasonal shift in Culex feeding from competent avian hosts to incompetent human hosts, and a consequent rise in the number of human infections by WNV (Kilpatrick et al. 2006).
All observations and analysis in this section were performed in November and December of 2014 by Michael as a part of The Evergreen State College’s Ornithology program.
Observation Narratives
11/9/2014
Mimirs Grove Farm
46.54.56N 122.53.59W
12:40-1:10pm
56°F, Partly Cloudy, Wind SW 17mph
I have noticed Robins congregating, sometimes in numbers exceeding a dozen, in the trees and field off of the southwest corner of the house, extending to the line of trees that borders a creek running N-S 30yds to the west of the house. On this occasion, notice a large flock of robins moving between the trees and the ground of the field, mainly keeping to the upper ¼ of tree branches. Observed 2 subjects in apple tree at height of 18ft, 5 ft short of the top. The other robins had moved toward the creek, and all birds were sharing space with a larger mixed flock that included numerous European Starlingst and Steller’s Jays, and at least one each Black-capped Chickadee, Song Sparrow, and Spotted Towhee. Many were keeping to the lower branches of the trees bordering the creek, obscured by brushy understory growth. Observed 1 Robin perched on 1/4in branch at height of 10ft over the creek, flies upward diagonally to perch 2ft higher in same tree, which has 1 other Robin in it that I can see. A Blue Heron flew overhead heading east. 1 Robin flies down to the right then left, landing on a branch at 7ft, and joined by another Robin on the same branch. Another Robin at 10ft leans down and brushes each side of its beak on the branch- I believe I observed this one other time. 1 subject flies down diagonally to 4ft. A distinct call is sounded, and all birds, Robins and otherwise, take off and fly, as though from danger, to the south. No raptor is observed, but a helicopter goes by overhead.
11/14/2014
Mimir’s Grove Farm, Orchard
46.54.59N 122.53.59W
8:45-9:30am
43 F, Clear, Wind SSW 8mph
First outing using rotating limited-time observation periods. Set interval timer to 1 min observations. Tracked mixed flock of Starlings, Robins, others from tall deciduous trees west of farmhouse to the apple trees of the orchard, about 80-100 ft NW of original perches. Managed to record 15 1-minute observations in 45-minute watch, mainly due to lack of familiarity with the process, fumbling between binoculars, notebook, and smart-phone interval timer. T. migratorius observed searching and foraging on hanging apples. Many simple gleans, many instances of reach-down gleaning from perch, very little to no other more acrobatic maneuvers such as hang-upside downs or lunges etc. Several instances of reach-down beak-wipes on the surface of the perch, one wipe on each side of the beak in quick succession. Have not learned to distinguish agonistic behavior.
11/17/2014
Mimir’s Grove Farm, Orchard
46.54.59N 122.53.59W
Mimir’s Grove Farm, Northeast Pasture and Evergreen Stand
46.54.60N 122.53.57W
10:00-11:30am
35 F- 43 F, Sunny, Wind ESE2 mph
Spent much of the morning trying to track Robins to various areas of the farm, locating them and then having them fly off to another area before I could take substantial notes. Continuing with time-limit observations of 1 minute duration before subject switch. A couple of observations in the orchard before tracking the flock to an area on the Northeast corner of the farm that includes pasture and some evergreen stands that they appear to favor. Observed several behaviors new to me. When perching in tall deciduous trees in or on the edge of pasture, they do not seem to be actively searching or foraging, and often preen. I spent time observing them below the canopy in the evergreen stand which did include understory deciduous trees, where they tended to actively search close to the trunk of the evergreens and probe the bark of the trunk, sometimes while briefly hanging upright from the bark of the trunk. On the ground beneath the redwoods, which was covered with dead deciduous leaves and needles, they actively searched and probed the ground, but did not seem to me to be doing the distinctive “head-cock” that they do when searching the ground of grassy pastures. I also observed their very active searching behavior on the ground of grassy pastures, where they regularly walk or hop along, stop, “head-cock” and glean-probe.
11/17/2014
Mimir’s Grove Farm, Northeast Pasture and Evergreen Stand
46.54.60N 122.53.57W
3:00-4:30pm
49ºF, Sunny, Wind N 3mph
Returned to the area of highest activity in the morning and made very similar observations. Did see some possible agonistic behavior with one perched bird sending a distinct alarm call and fluttering for cover just as another bird flew quickly toward the first bird’s original perch. Also saw 1 bird perched high in a deciduous tree regurgitate, and one bird searching in grassy pasture regurgitate a very small round object.
11/20/2014
Nisqually Wildlife Refuge, Parking Lot
47.4.21N 122.42.50W
11:30am-12:00pm
45ºF, Cloudy, Wind WSW 7mph
On our last field trip, searched fruitlessly for robins along the boardwalks running through the wooded marshy areas of the refuge, as well as in the grassy areas surrounding the barns. I felt that the habitat was not quite right, there are fruit bearing trees, but the ground is waterlogged. Also directly next to the sound, and saltwater. Eventually caught sight of several robins in trees at the edge of the parking lot, made a few observations, including I believe the first time I had seen what was unmistakably agonistic behavior between two robins, in which one clearly chased away another.
11/28/14
Mimirs Grove Farm, in and around orchard
10:15am-11:15am
Intermittent light rain, 47ºF
Now having a lot of trouble finding groups of Robins of any size, mostly have to search around for individuals and pairs. I feel as though the main migration from the farm may have happened, and have learned through my research that only a very small percentage of a local population will be non-migratory, though some birds also migrate to this area and winter here. Birds have become less tolerant of my approach. I have been trying to figure out where they are hunkering down when the weather is cold and rainy. I suspect that they may spend time in some of the many thick, impenetrable blackberry bushes around the farm. Having learned that Robins will sometimes gorge on fermented berries until inebriated, sometimes to the point of unconsciousness, I can’t help but imagine debauched scenes of overindulgence taking place in those berry bushes, Robins passed out drunk on the ground safe from my prying eyes. I am surprised not to find them foraging on the plentiful apples still on the branch in the orchard, and fear that I will have considerable trouble making observations of this diminished population.
11/29/2014
10:00am
Mimirs Grove Farm
35ºF, Sunny
Despite the sun being out, and much searchin, only observed a few individual Robins. Really think the bulk of the population has migrated away.
11/30/2014
9:15-9:30am
Mimir’s Grove Farm, East of house, 46.54.56N 122.53.59W
24ºF, Sunny, Wind ENE 8mph
Saw a Robin out the window on my way down to breakfast and headed out to make observations, 200ft East of house in and around dilapidated greenhouse structure. Was surprised to find several Robins foraging on the ground, despite the cold temperature and frost on the ground. Imagined that they had made the transition to an almost exclusive fruit and berry diet by now. Made me hopeful for further observation today. Continued 1-minute observations of the few Robins present, then headed back in to dress and eat before coming back out.
11/30/2014
10:00am-11:00am
Mimirs Grove Farm, in and around orchard
46.54.59N 122.53.59W
26ºF, Sunny, Wind ENE 8mph
Relieved to find a good number of Robins, though rarely more than one or two at a time, making 1-minute observations inefficient. In light of diminished numbers, may apply statistical analysis to existing data and do longer focal watches from here on out. Witnessed gleaning on berries for the first time, mostly had seen only foraging on ground for worms and arthropods, or in appletrees. In other deciduous trees I had only seen them perch and preen, usually at greater heights. Saw an adult male try unsuccessfully to eat berries in one tree, plucking them and holding them in bill repeatedly but then dropping them. Seemed unusual, as research has shown that foraging is an acquired skill and juveniles are more likey to drop foods after gleaning. The berries were very cold and hard, maybe also just a bit too big? Birds are more spread out, and seem distinctly more wary than a couple of weeks ago, which is supported in the literature regarding their post-breeding season behavioral shifts.
All data and analysis by Michael Szetela, November-December 2014.
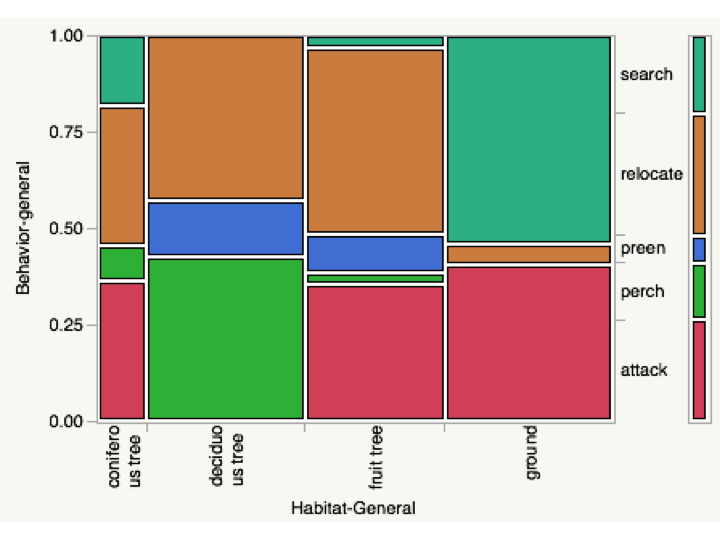
Data was collected at Mimir’s Grove Farm and the Nisqually Wildlife Refuge. Observations were one-minute rotating focal watches, quantifying habitat, microhabitat, behavior, and foraging. Data was analyzed with a Chi-squared test using JMP Pro to determine correlations between behavior and habitat. For the analysis habitats were generalized into four categories: coniferous tree, deciduous (non fruit-bearing) tree, fruit tree, and ground. Behaviors were generalized into 5 categories: search, relocate, preen, perch and attack. Search refers to active foraging searches. Relocate refers to any spatial movement, including flying, fluttering, hopping, and climbing. Preen refers to any preening activities including beak-wipes. Perch refers to perching in trees in the absence of any other activity during the focal watch limit. Attack refers to any active attempt to capture food, whether successful or not.
Chi-squared showed statistically significant correlations between habitat and behavior, but with 20% of squares having an expected count less than 5, making the test suspect.
High correlations were found between attack behavior and the habitats coniferous tree, fruit tree, and ground. Robins tended to spend time on the ground actively searching or attacking, and all spatial movement on the ground occurred in the course of actively searching. They were more spatially active, with high relocation, in coniferous, deciduous, and fruit trees, and did not attack while in non fruit-bearing deciduous trees.
Michael Szetela is a Sophomore at Evergreen pursuing his BA/BS in Latin American Studies and Evolutionary Theory.
Cancellieri, S. and M. T. Murphy. 2013. Experimental examination of nest reuse by an open-cup-nesting passerine: time/energy savings or nest-site shortage? Animal Behaviour 85:1287-1294.
Clucas, B. and J. M. Marzluff. 2012. Attitudes and actions toward birds in urban areas: Human cultural differences influence bird behavior. The Auk 129:8-16.
Croston, R. and M. E. Hauber. 2013. Spectral tuning and perceptual differences do not explain the rejection of brood parasite eggs by American robins (Turdus migratorius). Behav Ecol Sociobiol [Internet]. 2013 [cited 2013 November 23]. Available from DOI 10.1007/s00265-013-1649-8.
Eason, P. K., P. T. Sherman, O. Rankin, and B. Coleman. 2006. Factors affecting flight initiation distance in American Robins. Journal of Wildlife Management 70:1796-1800.
Eiserer, L. 1976. The American Robin. Nelson-Hall Inc., IL, USA.
Gill, F. B. 2007. Ornithology. W.H. Freeman and Company, NY, USA.
Govoni, P. W., K. S. Summerville, and M. D. Eaton. 2009. Nest sharing between an American Robin and a Northern Cardinal. The Wilson Journal of Ornithology 121:424-426.
Hamer, G. L., T. K. Anderson, G. E. Berry, A. P. Makohon-Moore, J. C. Crafton, J. D. Brawn, A. C. Dolinski, B. L. Krebs, M. O. Ruiz, P. M.Muzzall, T. L. Goldberg, and E. D. Walker. 2013. Prevalence of filarioid nematodes and trypanosomes in American Robins and House Sparrows, Chicago USA. International Journal for Parasitology, Parasites and Wildlife 2:42-49.
The IUCN Red List of Threatened Species. Turdus migratorius (American Robin). (n.d.).Retrieved December 1, 2012, from http://www.iucnredlist.org/details/106006444/0
Johnson, S. L. 2006. Do American Robins acquire songs by both imitating and inventing? The Wilson Journal of Ornithology 118:341-352
Kilpatrick, A. M., L. D. Kramer, M. J. Jones, P. P. Marra, and P. Daszak. 2006. West Nile Virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLOS Biology (Internet) April 2006 4(4)e82:0606-0610
Kroodsma, D. 2005. The Singing Life of Birds. Houghton Mifflin Company, NY, USA.
Kuehn, M. J., B. D. Peer, and S. I. Rothstein. 2014. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Animal Behaviour 88:21-28.
LaFleur, N. E., M. A. Rubega, and C. S. Elphick. 2007. Invasive fruits, novel foods, and choice: An investigation of European Starling and American Robin frugivory. The Wilson Journal of Ornithology 119:429-438.
Lampman,R. L., N. M. Krasavin, M. P. Ward, T. A. Beveroth, E. W. Lankau, B. W. Alto, E. Muturi, and R. J. Novak. 2013. West Nile Virus infection rates and avian serology in East-Central Illinois. Journal of the American Mosquito Control Association 29:108-122.
Lang, A. K., E. K. Bollinger, and B. D. Peer. 2014. Effect of parasite-to-host egg ratio on egg rejection by a Brown-headed Cowbird host. The Auk 131:694-701.
Lepczyk, C. A., K. G. Murray, K. Winnett-Murray, P. Bartell, E. Geyer, and T. Work. 2000. Seasonal fruit preferences for lipids and sugars by American Robins. The Auk 117:709-717.
Luning, E. (n.d.) (March 29, 2006). Robins in winter a harbinger of global warming, Audubon Society study says | The Colorado Independent. The Colorado Independent. Retrieved December 1, 2012, from http://coloradoindependent.com/21689/robins-in-winter-a-harbinger-of-global-warming-audubon-society-study-says
Miller, M. W. 2006. Apparent effects of light pollution on singing behavior of American Robins. The Condor 108:130-139.
Morneau, F., C. Lépine, R. Décarie, M. A. Villard, and J. L. DesGranges. 1995. Reproduction of American Robin (Turdus Migratorius) in a suburban environment. Landscape and Urban Planning 32:55-62.
Oyugi, J. O., and J. S. Brown. 2003. Giving-up densities and habitat preferences of European Starlings and American Robins. The Condor 105:130-135.
Rasmussen, J. L., S. G. Sealy, and T. J. Underwood. 2009. Video recording reveals the method of ejection of Brown-headed Cowbird eggs and no cost in American Robins and Gray Catbirds. The Condor 111:570-574.
Rowe, K. M. C., and P. J. Weatherhead. 2009. A third incubation tactic: Delayed incubation by American Robins (Turdus Migratorius). The Auk 126:141-146.
Rowe, K. M. C., and P. J. Weatherhead. 2011. Assortative mating in relation to plumage traits shared by male and female American Robins. The Condor 113:881-889.
Sauer, J. R., J. E. Hines, J. E. Fallon, K. L. Pardieck, D. J. Ziolkowski, Jr., and W. A. Link. (2011). The North American Breeding Bird Survey, Results and Analysis 1966 – 2010. Version 12.07.2011 USGS Patuxent Wildlife Research Center, Laurel, MD
Sibley, David, Chris Elphick, and John B. Dunning. (2001) The Sibley Guide to Bird Life & Behavior. New York: Alfred A. Knopf. Print.
Simpson, J. E., P. J. Hurtado, J. Medlock, G. Molaei, T. G. Andreadis, A. P. Galvani, and M. A. Diuk-Wasser. 2011. Vector host-feeding preferences drive transmission of multi-host pathogens:West Nile virus as a model system. Proceedings of the Royal Society B (Internet) 279:925-933.
Stokes, D. W. 1979. A Guide to Bird Behavior Volume I. Little, Brown and Company, MA, USA.
Sutton, G. M. 1986. American Robin (Turdus Migratorius) in Birds Worth Watching. University of Oklahoma Press, OK, USA.
Tait, M. 2005. Who was killed by cock robin? The Ecologist September 2005.
VanDalen, K. K., J. S. Hall, L. Clark, R. G. McLean, and C. Smeraski. 2013. West Nile Virus infection in American Robins: New insights on dose response. PLoS ONE (Internet) 8:e68537.
Vanderhoff, E. N. and P. K. Eason. 2007. Disparity between adult and juvenile American Robins Turdus Migratorius foraging for ground invertebrates and cherry fruits. Ethology 113:1212-1218.
Vanderhoff, E. N. and P. K. Eason. 2008. Comparisons between juvenile and adult American Robins foraging for Mulberry fruit. The Wilson Journal of Ornithology 120:209-213.
Vanderhoff, E. N. and P. K. Eason. 2008. Influence of environmental variables on foraging by juvenile American Robins. J. Field Ornithol. 79:186-192.
Vanderhoff E. N. and P. K. Eason. 2008. The response of American Robins (Turdus migratorius) to aerial alarm calls. Behaviour 146:415-427.
Vanderhoff, E. N. and P. K. Eason. 2009. American Robin seet Calls: Aerial alarm or a contact call? The Wilson Journal of Ornithology 121:406-411.
Warkentin, I. G., J. M. Reed, and S. M. Dunham 2003. Nest site characteristics of American Robins breeding in desert-riparian habitat. The Wilson Bulletin 115:16-23.
Wauer, R. H. 1999. The American Robin. University of Texas Press, TX, USA.




Leave a Reply