I lose access to this page on November 17, 2018 and this page may be deleted. My research has been moved to this site. I apologize for the inconvenience.
 © 2004 MBARI
© 2004 MBARI
ABSTRACT
Vampire teeth: Radula morphology of the vampire squid, Vampyroteuthis infernalis, indicates a soft-bodied diet
The feeding habits of Vampyroteuthis infernalis have been investigated through gut sample analyses, shipboard observations, in situ observations and stable isotope analyses. These observations have indicated a variety of food items including copepods, shrimps, squids, cnidarians and marine snow. Cephalopod radulae come in two basic forms: homodont with a single cusp on all teeth and heterodont that has more than one cusp on the rhachidian tooth and/or several teeth. Using light and scanning electron microscopy, the morphology of the vampire squid heterodont radula was compared to radulae of other cephalopods, including the Humboldt Squid, Dosidicus gigas, California market squid (Doryteuthis opalescens) and other midwater species. Scanning electron micrographs of radulae from over 15 species of cephalopods are compared in this study. Vampire squid have a unique homodont radula with a single cusp on all seven teeth. In comparison to the robust cutting heterdont radula and rhachidian teeth of shallow predators, the radula of V. infernalis has long gracile teeth that appear to be well suited to tearing apart very soft tissue and perhaps picking apart marine detritus. Despite living in extremely low oxygen concentrations with very low metabolic rates, adult vampire squid can be fairly active swimmers and likely feed on a variety of soft-bodied organisms.

Vampire squid illustration by Michael Cole, Oregon Coast Aquarium.
Introduction
Most molluscs have a radula which is a specifically unique feeding organ that serves as a grinding mechanism to tear apart food into smaller pieces. In cephalopods, the radula consists of symmetrical rows of 7-9 teeth. The radula is most often studied using a scanning electron microscope (SEM) to observe the three-dimensional relationships between the rows of teeth.
Towards the front end of the radular ribbon, the teeth become worn in feeding and are replaced by lower teeth continuously forming in the radular sac. The whole ribbon moves forward while modified odontoblasts dissolve and absorb older teeth and membranes. This movement was compared by Huxley (1853) to a chainsaw, with backward movement of the ribbon thrusting food into the pharynx.
There are two basic forms of cephalopod radulas, the homodont radula with a single cusp on all teeth which are similar in form across a row and the heterodont radula that has more than one cusp on the rhachidian tooth and/or several teeth. Form and shape of molluscan radular teeth are commonly exclusive to a species or genus. Each row of radula teeth consists of one rhachidian tooth followed by one or more lateral teeth on each side, and then one or more marginal teeth.
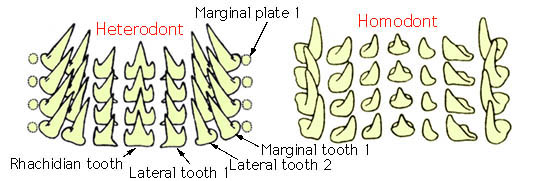
(taken from the Tree of Life Web Project – Cephalopod Radula page)
SEM photographs were taken to further study the radula in detail. Several methods are available in regards to preparing and mounting the radula of several mollusc species for SEM observations, but only one in specific detail about cephalopods. Zheng (2002) was the primary resource of this paper.
Materials and Methods
Radulae or buccal cavities were retrieved from three cephalopods. Two radulae from Dosidicus gigas were given by the Gilly Lab from Hopkins Marine Station of Stanford University. Vampyroteuthis infernalis was given by Dr. Erik Theusen at The Evergreen State College. Doryteuthis opalescens was self-collected in San Francisco, CA.
The radulae were observed under the scanning electron microscope using the following protocol, Zheng (2002): radula was washed with diluted water three times in order to wash away food residue and other adhesive materials. The tissues were immersed into a 1% KOH solution for 24 hours, where specimen were then prefixed for 12 hours in 3% glutaraldehyde at 4°C.
After being washed with diluted water three times, the tissues were dehydrated rapidly through a graded ethanol series (30, 50, 70, 80, 90, 95%) for 10 minutes each, and placed through a grade isoamyl acetate series. The tissues were CO² critical point dried, and coated with gold. They were then examined with the JEOL JSM-6480LV scanning electron microscope. Some specimen were examined using the Z-stacking stereo microscope Leica MZ16.
Through emailing researchers and finding what is local, 15 species were prepared over the course of my research.
Results
Vampyroteuthis infernalis Vampire squid
Euprymna scolopes Hawaiian bobtail squid
Sepia bandensis Dwarf cuttlefish
Sepia officinalis Common cuttlefish
Nautilus pompilius Chambered nautilus
Sepioloidea lineolata Striped pyjama squid
Metasepia pfefferi Flamboyant cuttlefish
Dosidcus gigas Humboldt squid
Doryteuthis opalescens California market squid
Octopus bimaculoides California two-spot octopus
Octopus briareus Caribbean reef octopus
Octopus hummelincki Caribbean two-spot octopus
Doryteuthis pealeii Longfin inshore squid
Taonius borealis Translucent cockatoo squid
Enteroctopus dofleini Giant pacific octopus
ADDITIONAL PHOTOS FROM MY RESEARCH
OTHER RESEARCH ON OTHER CEPHALOPOD RADULA
OTHER RESEARCH ON OTHER MOLLUSCA RADULA
ACKNOWLEDGEMENTS
I would like to thank Dr. Erik Thuesen for sponsoring my research project to further my knowledge of SEM techniques and cephalopods, as well as for donating Vampyroteuthis infernalis and Taonius borealis from a MBARI Research cruise.
I thank Patrick Daniel from the Hopkins Marine Station of Stanford University for responding to my inquiries about specimen needed and donating the radulae of Dosidicus gigas from their Bay Area Science Festival’s Discovery Day display at AT&T Park in San Francisco, CA.
From Professor David Sheels lab at Alaska Pacific University in Anchorage, I thank Paul Bennetts for dissecting and sending buccal cavities of Enteroctopus dofleini.
I would like to thank Denise Whatley and Tonmo for donating Octopus briareus, Nautlius pompilius, Sepia bandensis and Octopus hummelincki.
I also thank Eric Koch, a PhD student in the lab of Margaret McFall-Ngai/Edward Ruby for catching Eupyrmna scolopes in the shallow sand flats of Oahu and sending several samples to me.
From the “Cephalopod Breeding Initative” of Marine Biological Laboratory in Woods Hole, Massachusetts I received the buccal cavities of Sepia officinalis, Sepia bandensis, Doryteuthis paeleii, Octopus bimaculoides, Euprymna scolopes, Sepioloidea lineolata and Metasepia pferfferi. Thank you Lisa Abbo for performing the dissections, Roger Hanlon’s lab for providing the Sepia officinalis samples, and Bret Grasse’s cephalopod culture lab for providing all the other specimens.
And finally I would like to thank my parents for helping me find several specimen of Doryteuthis opalescens and teaching me how to cook squid adobo.
O’Shea, Steve, & Lu, C.C.. (2002). A New Species of Luteuthis (Mollusca: Cephalopoda: Octopoda: Cirrata) from the South China Sea. Zoological Studies, 41(2): 119-126
References
- Hoving, H. J., & Robison, B. H. (2012). Vampire squid: detritivores in the oxygen minimum zone. Proceedings of the Royal Society of London B: Biological Sciences, 279 (1747) 4559-4567
- Hoving, H & Perez, Jose & S.R. Bolstad, Kathrin & Braid, Heather & Evans, Aaron & Fuchs, Dirk & Judkins, Heather & Kelly, Jesse & Marian, José & Nakajima, Ryuta & Piatkowski, Uwe & Reid, Amanda & Vecchione, Michael & Xavier, J. (2014). The Study of Deep-Sea Cephalopods. Advances in marine biology. 67C. 235-359. 10.1016/B978-0-12-800287-2.00003-2.
- Messenger, J. B. (1999). The radular apparatus of cephalopods. Philosophical Transactions of the Royal Society B: Biological Sciences, 354(1380), 161–182.
- Pickford, G.E., (1946). Vampyroteuthis Infernalis Chun, An Archaic Dibranchiate Cephalopod. C.A. Reitzels Forlag.
- Samuel, D. V., & Patterson, J. (2003). A comparative study on the radula of three Coleoid Cephalopods. South Pacific Study, 24(1), 33-38.
- Stewart, J. S., Hazen, E. L., Bograd, S. J., Byrnes, J. E., Foley, D. G., Gilly, W. F., … & Field, J. C. (2014). Combined climate‐and prey‐mediated range expansion of Humboldt squid (Dosidicus gigas), a large marine predator in the California Current System. Global change biology, 20(6), 1832-1843.
- Siebel, Brad A., Chausson, F., Lallier, Francois H., et al., (1999). “Vampire Blood: Respiratory Physiology of the Vampire Squid (Cephalopoda: Vampyromorpha) in Relation to the Oxygen Minimum Layer,” Experimental Biology Online 4, no. 1: 1-10
- Siebel, Brad A., Hafker, N. S., Trubenbach, K., et al., (2014). “Metabolic Suppression During Protracted Exposure to Hypoxia in the Jumbo Squid, Dosidicus gigas, Living in an Oxygen Minimum Zone,” journal of Experimental Biology 217, no. 14: 2555-2568.
- Siebel, Brad A., Thuesen, E. V., Childress, J. J., (1998) “Flight of the vampire: ontogenetic gait-transition in Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha) Journal of Experimental Biology, 201 (16): 2413-2424.
- Uyeno, T. A., & Kier, W. M. (2005). Functional morphology of the cephalopod buccal mass: a novel joint type. Journal of morphology, 264(2), 211-222.
- Venkatesan, V., Ramesh Kumar, P., & Babu, A. (2016). Scanning electron microscope studies on the radula teeth of four species of marine gastropods from the Gulf of Mannar, India. Indian Journal of Fisheries, 63(1), 140-145.
- Villanueva, R., Perricone V., Fiorito G., (2017). Cephalopods as Predators: A Short Journey among Behavioral Flexibilities, Adaptations, and Feeding Habits. Front Physiol. 2017; 8: 598.
- Young, R.E., Vecchione, M., Mangold, K.M. (2000) Cephalopod Radula. Tree of Life Web Project.
- Zheng, X. (2002). dong, Wang, Ru- cai., Ocean University of Qingdao, Qingdao266003, China); Morphological study on radula of nine cephalopods in the coastal waters of China [J]. Journal of Fisheries of China, 5.
- Scanning Electron Microscopic Studies on Radula

