What *Wood* A Fungus Eat
What *Wood* A Fungus Eat?
Daniel Steelquist steelwhitepine@gmail.com
Cody Allison codyma3@gmail.com
Daniel Gilberts dhgilberts@aol.com
Fungal Decomposition
How are fungal decomposition rates affected by different wood substrates compared to the absence of macro fungi in a control? How might this affect plant growth in surrounding areas in a variety of habitats?
Summary
By observing rates of decomposition for Pleurotus ostreatus on various Cherry, Mesquite, and Alder woods as similarly found in various habitats we hope to understand how fungal decompositon might help plants grow.We wonder what implications the decomposition and rate of mycelial growth have on renewable resource management and applications within the greater issue of climate change.
[1]Mycoremediation and mycoforestry are applications in which this information may be directly utilized. Mycoremediation is the use of fungi to degrade or remove toxins from the environment. Mycoremediation involves the use of mycelial mats to help break down complex toxins into similar less-toxic substances. Methods of pulling environmental toxins from polluted water ways and soils, especially in inner city areas, can draw from our research more information as to which wood might be best suited for the applications. Mycoforestry is the use of fungi to aid in the health of forests and can be used to help preserve native forests, recycle woodland debris, and help give replanted trees a better chance of survival. In forestry applications dealing with clear cut areas, workers can consider use of Pleurotus ostreatus in breaking down woody debris in conjunction with plantings of saplings to help speed up the new forest growth and ease the disturbance of harvesting resources, especially lumber.
Intro/Background
Our group developed an experiment to determine what type of wood is decomposed the quickest by Pleurotus ostreatus (the tree oyster mushroom).

1.Our hero: Pleurotus ostreatus (Tree Oyster Mushroom) photo credit: voir ci-dessous
[2]The “pleuro” in Pleurotus means sideways positioned and represents the mushroom growing laterally like shelves from the material it grows upon. The ostreatus refers to the color and shell-like shape of its mushrooms that resemble an oyster.
[1]Most of the fungi that we eat are considered saprophytic which refers to their favoring of decomposing wood. These fungi are responsible for the major part of recycling on our planet and are of major importance to all of our ecosystems. They secrete acids and enzymes that break down the large molecules that plants are composed of into smaller compounds and make them more accessible to neighboring organisms. Fungi function to return carbon, hydrogen, nitrogen, and minerals back into soils. Some fungus species can have many roles in an environment as is Pleurotus ostreatus, the main subject of this article.
[1]This mushroom peaked our interest after reading about many of the fascinating attributes of the species. It has inspired many new fungal technologies for pulling toxins out of ecosystems (bacteria, heavy metals, PCBs, hydrocarbons, etc), to grow as a sustainable food source, to pull oil from oil spills in the ocean, to improve the health of forests and aid in their recovery as well as many other uses. This fungal ally and other related species in the Pleurotus genus are also very common in areas all over the United States and is one of the first fungi you see growing on fallen wood debris of deciduous trees in forests. The culture used for this project was grown from a mushroom that was gathered during a mushroom foray in Capital Forest near Olympia Washington.
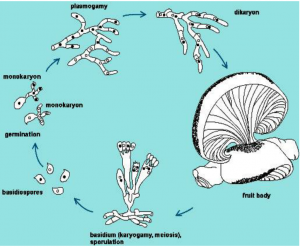
2.Life Cycle of Pleurotus Ostreatus by Martínez-Carrera
[2]Pleurotus ostreatus is one of these wood decomposing fungi typically found growing in hardwood river valleys. Although this is typically the case, these fungi are known to be highly adaptable. In an instance where spores from a Pleurotus florida (a very close cousin species) were crossed with Pleurotus ostreatus, the mycelium formed connections and grew to eventually fruit mushrooms. [5]This ability to be able to mingle with closely related species might be a source of its adaptability. Pleurotus ostreatus can be seen to be extremely successful evolutionarily as it grows on a wide diversity of hardwoods including cottonwoods, oaks, alders, maples, aspens, ash, beech, birch, and many other trees. It can also be found growing on conifers but is less common.
[7]This fungus is also considered a predaceous fungi and actively seeks out nematodes in order to consume the rich nitrogen they contain as their primary food source, wood, is very low in it. They have developed mechanisms for trapping the nematodes and anesthetizing them with their hypae(web-like branching growth). They eventually suffocate the tiny worms then grow into their organs, break them down by excreting enzymes, and absorb the nutrients.
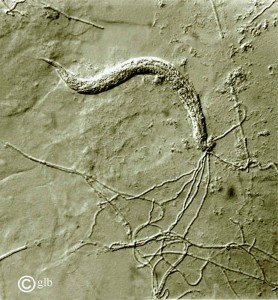
3.Pleurotus ostreatus vs. nematode
Image by George Barron.
[5]Pleurotus genus fungi have adapted to more than justas primary saprophytes, or the first to begin breaking down wood and other materials. They have also developed in order to take advantage of weakened trees that have been attacked by disease, bacteria, other fungi and organisms, and environmental factors such as storms and wildfires.[1] In this case it can also be called a parasitic fungi. They will continue to grow even after that host tree has died, but typically will only take advantage of parasitism when the ecology around them is changing more rapidly and they have a lack of steady nutrients from more common sources.
[2]The tree oyster fungus can grow on a wide variety of other materials because it has a wide range of pH in which it inhabits from pH of 5.0 to 8.0. It has been thought of as a secondary crop for various materials from industries such as straw production on farms, agave, soybeans, bananas, coffee, mangoes, dates, sugarcane, barley, rye, and cornstalks. It also has been used to reduce the contamination of byproducts from these industries such as the elimination of toxic caffeine by up to 90% from getting into the environment from coffee production.
With this fungus being known for its rigorous growth we wondered if the type of wood would greatly effect the expansion of mycelium, the root-like structures of the fungus. In order to find out if there was a difference in growth determined by the difference in wood we measured decomposition rates compared to original mass with a mass analysis.
Hypothesis–
The time for decomposition of Pleurotus ostreatus in wood is determined by the type of tree it came from. Because of its high level of adaptability, the Pleurotus ostreatus should be able to breakdown hardwoods from a variety of habitats.
Methods
[1]According to Paul Stamets’ Growing Gourmet and Medicinal Fungi, the prime growing parameters for Pleurotus ostreatus mycelium is as follows:
Temperature: 75F
Relative Humidity: 85-95%
Duration: 12-21 days
CO2:5000-20,000ppm
Fresh Air Exchanges: 1 per hour
Light requirements:n/a

4.Our three wood species
In our experiment we are determining the decomposition factors affecting the growth rate of Pleurotus ostreatus on our three types of wood substrates of Cherry, Mesquite, Alder.

5.Filling bags with wood and mycelium
We filled our “filter bags” with wood and drew on with a sharpie a “site window” each 4cm x 6cm on the side of the bag. To see growth photos were taken daily within the “site window” for consistency.
Sterilization of wood substrates- Wood substrates were placed into sealed Sterilite containers with a 50% Hydrogen Peroxide/ 50% water mix for 48 hours to ensure substrates are sterile and hydrated for mycelium to be added(inoculation).
2 Controls- Rye grain w/mycelium and Cherry wood
3 wood Substrates- wood substrates are chipped
- 3 bags (1,2,3) of Cherry Wood @1.5 lbs/0.3125 lbs Myceliated rye
- 3 bags (1,2,3) of Mesquite @1.5 lbs/0.3125 lbs Myceliated rye
- 3 bags (1,2,3) of Alder @1.5 lbs/0.3125 lbs Myceliated rye
A fruiting body of the Pleurotus ostreatus fungus (oyster mushroom) collected from the Capital Forest near Olympia, WA growing on a fallen Alder log. Spores were collected on a piece of paper after letting the mushroom sit over night. A flask of potato dextrose agar was set in an autoclave for 30 minutes at 15 PSI. The agar was cooled for 2-3 hours. HEPA flow hood was turned on 30 minutes prior to doing work in lab space and all materials were set out to be used. Agar was poured into a set of 10 petri dishes. One by one the spores were taken from the piece of paper and gently swiped across the agar in a technique called streaking. Each petri dish lid was opened towards the flow hood and all actions by hand or tool downstream to the dish in order to prevent contamination. Mycelium was grown on petri dish of media over the course of 2 months.
Example of mycelium expanding on agar in petri dish
Rye grain was prepared via soaking in RO water for 24 hours before mycelial transfer. The rye was collected into quart jars to fill 3/4 of the jar. Special filter lids were placed over the top which allow gas exchange while the mycelium expands. These filters are comprised of a double layer of tyvek cut into circles, a central hole, and micropore tape over the top of the hole with the silver, reflective side facing down and the internal white part facing up. Aluminum foil sheets were cut to cover lid in order to limit water loss in the jars. The jars were set into the autoclave for 1.5 hours at 15 PSI and left to cool over night. The chosen agar dish with mycelium was set up in front of the flow hood once again 30 minutes before hand and sterilized using isopropyl alcohol. Sections of the outermost(newest) growth were used to ensure viability and cut into close to 3mmx3mm sections and transferred to the sterilized rye grain with attention to the air stream and making sure contaminants didn’t make their way in. The jars were left to grow out for three weeks.
Example of mycelial growth on grain in jar. In this case it is growing through Trichoderma Sp. mold
Wood was sourced from the Bark Center for landscaping purposes. We chose Alder, Cherry, and Mesquite wood chips of about the same size. The wood was separated into containers and for a Hydrogen Peroxide soak for 48 hours. We drained the Hydrogen Peroxide and prepared perforated micron filter bags specifically designed for fungal cultivation. We transferred in the open air 1.5 lbs of each wood and 5 ounces of myceliated rye to each bag, weighing with just wood and then with the mycelium. We sealed each bag using an impulse sealer and labeled bags M1,M2,M3,C1,C2,C3,A1,A2,A3, mycelium control, and wood control of cherry. The bags were placed on racks within a grow tent and we photographed the expansion of growth every day.
Bag rotation once daily to different places on racks in order to ensure even light exposure and temperature consistency.
There was a constant light exposure from 60 watt iridescent bulbs of 575 Lux measured on Labquest.
Our temperature range was 62-77 degrees F taken twice daily morning and evening on a digital thermometer.
Here is our footage of the mycelium control bag and best performing bag:
We opened the bag of mesquite and took a colonized chip and compared it to a chip in our wood chip control:
Weight of chips before dehydration:
Control:2.7g
Myceliated:10.6g
Weight of chips after dehydration:
Control:2.5g
Myceliated:6.1g
We brushed mycelium off of the surface of dehydrated chip:
mycelium portion:1.3g
wood portion:4.8g
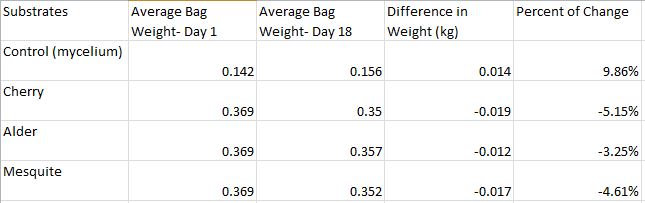
(Percent of change in above chart represents the change in weight between day 1 and day 18. The control of cherry wood was not included in this chart because there was no change in weight. The graph below illustrates no change in cherry control chip weight.)
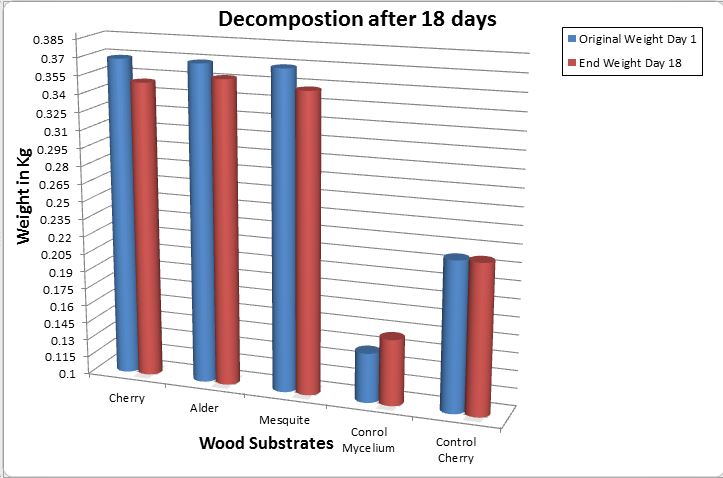
(Graph above illustrating the change in weight between all substrates and controls. control 1 with only mycelium. Control 2 with only Cherry wood chips. All wood substrates inoculated with mycelium on day 1 of the experiment. There was no change in weight within the cherry control bag.)
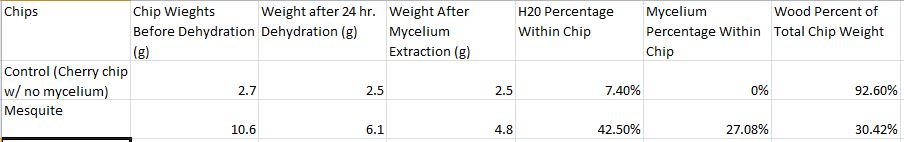
(Chart above illustrating the percentages of water/mycelium/wood within an average myceliated mesquite chip and average control chip.)
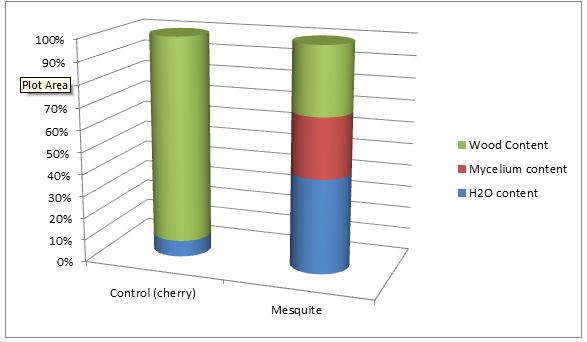
(Graph illustrating the water/mycelium/wood content percentages between an average myceliated mesquite chip and average control chip.)
Results:
Our Cherry bag control actually developed a mold which might be some extremophile endophyte[10](organism that can withstand intense situations inside another organism) living within the wood prior to Hydrogen Peroxide soak that was able to survive. We saw no change in the weight of the control and only observed minor spotting of mold growth.
In our mycelium control bag, we observed an increase in weight from 5g to 5.5g which was the greatest increase in mass seen in any of the bags. We believe that this is a perfect example of the actual function of mycelium in an ecosystem. The mycelium works as a sponge. As this fungus was continuing to consume the rye that it was originally inoculated to, instead of dehydrating, it actual seemed to draw moisture from the environment outside of the bag. This function of mycelium is currently being explored by researchers studying the role of fungi in weather creation and alteration. In their research, the spores are seen as the active mechanism for creation of rain clouds.[9] We wonder if it’s not only the mushroom that effects the weather, but that the mycelium could actually be helping to attract moisture to substrates in order to create enough humidity in the air around it to fruit. This activity has actually been observed as being a core part of the Pleurotus ostreatus life cycle. This fungus actually initiates fruiting as soon as the substrate in which it is growing on is fully colonized and covered with it. This strategy in quickly accumulating moisture probably accounts for how efficient it is at expanding through an environment. It can quickly consume material and form mushrooms that open and release its reproductive spores to move to another substrate without wasting any time.
We observed that the Pleurotus ostreatus from our local area actually preferred Mesquite wood from the Southwestern United States. It actually had almost completely colonized the Mesquite within our 18 day data collection. We wonder if its nitrogen fixation played a role in greater levels of nitrogen existing within its wood aiding in quicker mycelial growth.[11] This also goes against research that we have seen with untreated Mesquite in which it was considered an inferior substrate for Pleurotus ostreatus growth.[8] Is it possible that a bacteria that is present within the wood was killed during our Hydrogen Peroxide soak and the fungus consumed it or at least did not have to fight it which led to speedier growth? We also observed that this wood allowed the mycelium to actually pull moisture from outside the bag through the filter and into the bag increasing the over all weight. One mesquite bag exhibited this behavior whereas the only other bag to do this was the control of mycelium with the same increase in weight result.
In our Cherry bags we observed a bacteria develop and restrict the growth of Pleurotus ostreatus. This was strange as Cherry has been a fairly consistent substrate for mycelial growth. These bags also constituted the most reduction on average of mass. We wonder if the quick life span and reproduction of bacteria could explain the moisture effectively being “burned off” through speedy multiplication?
In our Alder bag we were surprised to see this being the least changed of the samples. This was the original type of wood that the original mushroom was found upon. We had assumed that the Alder would show the most significant change, however, it did grow but lagged behind the Mesquite. We wonder if the possibility of increase in nitrogen due to Mesquite being a nitrogen fixing tree might have given it an edge over the Alder.[3] Another possibility is that this fungus could have gotten a vigorous surge in adaptability when applied to a compatible wood that it never had the option of consuming before.
Through our experiment we saw numerous anomalies that developed further points of inquiry. Although we did develop quite a bit of data on decomposition of Pleurotus ostreatus, we developed many more questions that will continue our research into the future to discover more about the mysterious roles these fungi have on ecosystems.
Further questions:
Could movement of the bags greatly change air exchange?
How would more or less temperature flux affect the growth of Pleurotus ostreatus on various substrates?
How adaptable is Pleurotus ostreatus when moving from one type of substrate (grain) to another (wood, husks, straw, etc)?
How will supplementation of various additives ie. rice bran, oat bran, gypsum, lime, etc. change the growth rate?
What affect does the size of the grain used to inoculate the wood have on expansion of mycelium?
What affect does the Pleurotus ostreatus have on various materials and their structural integrity on the final myceliated mass? (building material applications)
What gas does the fungus give off during the colonization process? Would different materials affect the composition of the gas? Does this have viable applications in the biofuel industry?
We believe that if there had been a control with each type of wood, we may have been able to see a more accurate change in hydration and mass conversion of material to fungal growth.
In future experiments we plan to test growth on more types of wood and substrates in order to determine more of what factors led to the data we observed in this experiment. We hope that in continued testing we can find a pattern of continuing results such as the fungus at a certain temperature grows healthy and quickly on a certain substrate compared to others. We also hope to better understand what mechanisms lead to expansive growth and what inhibits the growth.
[5]Digging deeper it may be possible to adapt this information in areas such as biofuel production and alternative building and packing material creation. [6]This could also give us a lens as to which species might be best suited to certain areas in regrowing harvested timberland and recovery from wildfires. Understanding what materials grow the quickest could lead us to medicine and food from storm debris following stom surges or teach us about how to attract moisture to desert regions. We can learn which materials seemed to sustain the growth the longest which might be useful in biofilter applications to absorb heavy metals and hydrocarbons from polluted waters. The wonderful thing about fungi is there is such little we know about them. There are surely surprises in future research to be found and we invite you to replicate or expand upon our experiment in order to get better acquainted with these organisms. One thing is for sure, the future is fungi!
BIBLIOGRAPHY
- Stamets P. Growing Gourmet and Medicinal Mushrooms. Berkeley, CA: Ten Speed. [Print] 1994
- Sparks, Grace Beehler. Influence of Forest-clearcut Edges on Fungal Fruiting, Litter Decomposition and Seedling Growth in Low Elevation Second-growth Conifer Forests in Western Washington. [Print] 2003
- Franklin E. R. Eichhorn S. E. Raven P. H. Raven Biology of Plants [Print]2013
- McCoy P. Radical Mycology: A Treatise on Seeing & Working with Fungi. [Print] 2006
- Rayner A. D. M., and Boddy L. Fungal Decomposition of Wood: Its Biology and Ecology. Chichester: Wiley, [Print] 1988
- Jackson L. UA Campus Repository. The Safety and Efficacy of Pleurotus ostreatus (Oyster Mushroom) Cultivation on Prosopis spp. Products [Internet]. 2015 [cited 2016 May 13]. Available from: http://arizona.openrepository.com/arizona/handle/10150/55697
- Hassett MO Fischer MWF Money NP. Mushrooms as Rainmakers: How Spores Act as Nuclei for Raindrops. Dartmouth. [Internet] 2015
- Cavicchioli R. Amils R. Wagner D. McGenity T. “Life and applications of extremophiles” Environmental Microbiology. [Internet] 2011. P1903-1907
- Geesing D. Felker P. Bingham R. L. “Influence of mesquite (Prosopis glandulosa) on soil nitrogen and carbon development: Implications for global carbon sequestration.” Journal of Arid Environments Vol.46, Issue 2. [Internet] 2000. P157-180
Picture references:
1.Our hero: Pleurotus ostreatus (Tree Oyster Mushroom) photo credit: Jean-Pol GRANDMONT
Pleurotus ostreatus. Wikipedia.org [Internet][cited 2006 Sept 13]. Available from https://commons.wikimedia.org/wiki/File:Pleurotus_ostreatus_JPG7.jpg
2.Life Cycle of Pleurotus Ostreatus by Martínez-Carrera
Pleurotus ostreatus Life Cycle. Martinez-Carrera [Internet] [cited 1999] Available from https://www.researchgate.net/figure/270510548_fig2_Figure-2-Life-cycle-of-the-oyster-mushroom-Pleurotus-ostreatus-Source
3.Pleurotus ostreatus vs. nematode Image by George Barron.
Pleurotus ostreatus vs. Nematode. Uoguelph.ca [Internet] [cited 2008]. Available from http://www.uoguelph.ca/~gbarron/2008/pleurotu.htm
4.Our three wood species
5.Filling bags with wood and mycelium
Video references:
1.Example of mycelium expanding on agar in petri dish
Glenn Coville. “Pleurotus ostreatus (Oyster Mushroom) mycelium growing in petri dish” Online video clip. YouTube. YouTube. [Web] 2013
2.Example of mycelial growth on grain in jar. In this case it is growing through Trichoderma Sp. mold
G’ Channel. “Pleurotus mycelium vs Trichoderma (Timelapse)” Online video clip. Youtube. [Web] 2013
3.Our video of a 15 picture time lapse of Pleurotus ostreatus on Mesquite