Forest Fungi – Favorable or Fiendish?
Are pathogenic fungi lurking in our Washington forests and if so, what are their impacts? What deadly creatures conceal themselves in the soil depths and creep beneath the roots of the trees we see every day? Thusly we began our quest to find these unknown terrors!
Let’s take a walk through the forest and see what ghastly rots may be in store. Imagine we are walking through the woods in western Washington. Just this once, it’s not raining. We start on the path at an ambling pace, with an eye out for anything interesting. What do you see? There are trees, shrubs, and the path in front of you. There is moss everywhere. To your left you can see a leaning snag sporting several hefty shelf mushrooms. To your right there is a sad looking western hemlock surrounded by the pervasive honey mushroom, Armillaria. The honey mushroom is a pathogen, and will eat its host to death while its root-like rhizomorphs creep toward neighboring trees.
A pathogen is an organism that causes disease in another living organism. The organism the pathogen parasitizes is called the host. Most types of living creatures including plants and animals can be host to pathogens. Some pathogens of forest plants form mushrooms, one of the more conspicuous signs of infection. Mushrooms, also known as fruiting bodies, are reproductive structures of fungi analogous to the fruit of an apple tree. Fruiting bodies are one way that fungi produce spores, reproductive structures that can drift through air or water, or just lay wait in the soil, eventually landing on and infecting a new susceptible host. Fungi can grow in soil and spread to roots, bark, and heartwood of trees, and not all produce fruiting bodies. For this reason, you might only be able to see symptoms of disease, such as the unhealthy yellowing of needles on this Douglas-fir beside the trail, or a healthy looking tree leaning in the midst of a canopy gap in the woods.
Our research on pathogenic fungi causes us to seek out these dejected trees. Our group works to collect, to preserve, to teach. We have sought information from pathogen experts throughout the area, who gladly helped us in our project to create a teaching collection for future students. We have been enthusiastically gathering mushrooms sprouting from trunks, yellowing needles with plugged stomata, hole-ridden roots and laminated annular rings. We poked, prepared, processed, took pictures, personified, pondered and persisted in our project. It soon became clear to see how important pathogen disease is in keeping the ecosystem of our forests balanced.
Fungi are able to grow swiftly, and can sometimes reproduce multiple generations within a matter of weeks. In contrast, trees take years to reach maturity, let alone reproduce, resulting in a timeline which severely curtails their ability to adapt to new stresses. Recent studies have predicted an increase in pathogen fruiting periods in response to warming, which will strain host species (Roy et al 2004 and Shafer et al 2001). An accelerating climate change coupled with monocrop-style forestry practices puts considerable stress on trees, which are then more susceptible to pathogen infection. Matters are further complicated by the disappearance of healthy forests as they are swallowed up by modern development. This combination of unprecedented climate change, industrialization, and advantageous pathogenic fungi creates the potential for a dramatic shift in Northwest forests.
In spite of all this doom and gloom, plants are typically equipped with the means to fend off hungry pathogens. Their cell walls are made of cellulose and lignin, which are tough protective materials. Plants synthesize antimicrobial secondary metabolites to battle threatening invaders and produce detoxifying enzymes to break down the compounds that pathogens use to weaken plant tissues. When sensing a fungal invasion, some plants develop a thick structure inside the cell to wall off the offending fungus and protect the cell. A plant will even kill off its own infected (and nearby healthy) cells in what is called a hypersensitive response. This prevents further infection and can be a small sacrifice for the overall health of the plant. Alas, despite these defensive efforts, many plants still yield to the relentless pathogenic pressure.

Swiss Needle Cast
Standing under the sad-looking western hemlock, you think a little bit more about the honey mushrooms consuming it. What will be the fate of this tree? What other pathogens grow in these woods? You notice the corky bark of a Douglas-fir. Its bark looks healthy, but the needles are turning a sickly yellow. Pull out your hand lens, you may be able to see the tiny black fruiting bodies of the culprit: Swiss Needle Cast (Phaeocryptopus gaeumannii) (Figure 1). This is one of the most common foliar pathogens in our area. Swiss Needle Cast’s damage is not just cosmetic. The fungus plugs up the stomata, small openings in the needles that act like little noses for the tree, and the tree can’t breathe well enough to photosynthesize properly.
You notice the tree next door has toppled over. Walk over and take a closer look at the decay. You see the roots still buried in the ground with the rest of the trunk cracked and broken off a few feet from the forest floor. Check inside the stump. You’ll see the inner layers of wood have “delaminated” and peeled apart to form separated layers like thick pages of a book. This is the work of Washington’s number one tree killer, Laminated Root Rot (Phellinus sulphurascens).
In the end, we began to realize fungal pathogens are actually a vital part of a healthy forest ecosystem. We needn’t be so harsh with our adjectives and peg creepy, sneaky, invading pathogenic fungi as a plague upon the forest. The truth is, they also enhance forest ecosystems by creating canopy gaps and increasing availability of nutrients to other organisms (Hansen et al 2000, Laine 2004). Our sad-looking western hemlock will eventually fall, but the honey mushroom will continue to feed on the tree and recycle the nutrients back into the forest. The gap left in the canopy will let sunlight reach understory vegetation, which will give rise to other species that had been competing for light. This hemlock is not alone in its fate. Death by fungi isn’t always pretty, but it has an important role in the forest. Through our research we realized that the plague begins when an altered environment allows pathogens to grow out of control and throw an ecosystem out of balance. Next time you set off down that open trail keep in mind the delicate balance of give and take in nature around you.
Sources:
Hansen, EM; Goheen, EM. 2000. Phellinus weirii and Other Native Root Pathogens As Determinants of Forest Structure and Process in Western North America. Annual Review of Phytopathology, 38: 515-39.
Laine, A. 2004. Resistance Variation within and among Host Populations in a Plant: Pathogen Metapopulation: Implications for Regional Pathogen Dynamics. Journal of Ecology, 92: 990-1000
Roy, B; Güsewell, S and Harte J. 2004. Potential Changes in the Distributions of
Western North America Tree and Shrub Taxa under Future Climate Scenarios. Ecology,
Vol. 85, No. 9: 2570-2581
Shafer, SL; Bartlein, PJ and Thompson, RS. 2001. Potential Changes in the Distributions of Western North America Tree and Shrub Taxa under Future Climate Scenarios. Ecosystems, Vol. 4: 200-215
Forest pathogen Music Mix
Don’t have time to read our posts and blogs? Just hit play and listen to the wonderfully talented Case Cogan read our research and descriptions over a trippy ambiant mycomusic soundscape! ENJOY!
Root Rot in the Evergreen Woods
Today I was walking along a trail through the woods near Evergreen State College. I came to an area where I noticed many large windthrown Douglas Fir trees, some ripped out of the earth at a snapped-off root crown , some with trunks cracked and broken a couple of feet above the ground. There was a gap in the canopy above me. I poked around some stumps and noticed cream colored mycelial pockets and white mycelial fans growing just beneath the top layer of bark. The roots themselves where rotted to a white spongy texture and were riddled with little holes. I looked around and noticed many other trees leaning to varying degrees, some a little bit and some quite a lot. After inspecting all the trunks I found conks of Fomitopsis cajanderi, a sometimes pathogenic and often saprobic fungus. But I don’t think this fungus was the culprit that rotted the roots of these mature Douglas Fir trees. This fungus probably colonized the log through stem and branch wounds soon after the trees fell. Noting the cream colored pockets of mycelium beneath the bark, the white spongy rotten roots riddled with small holes parallel to the grain, and the lack of any noticeable delamination of the wood I think Heterobasidion annosum is the root rot fungus present at this spot.
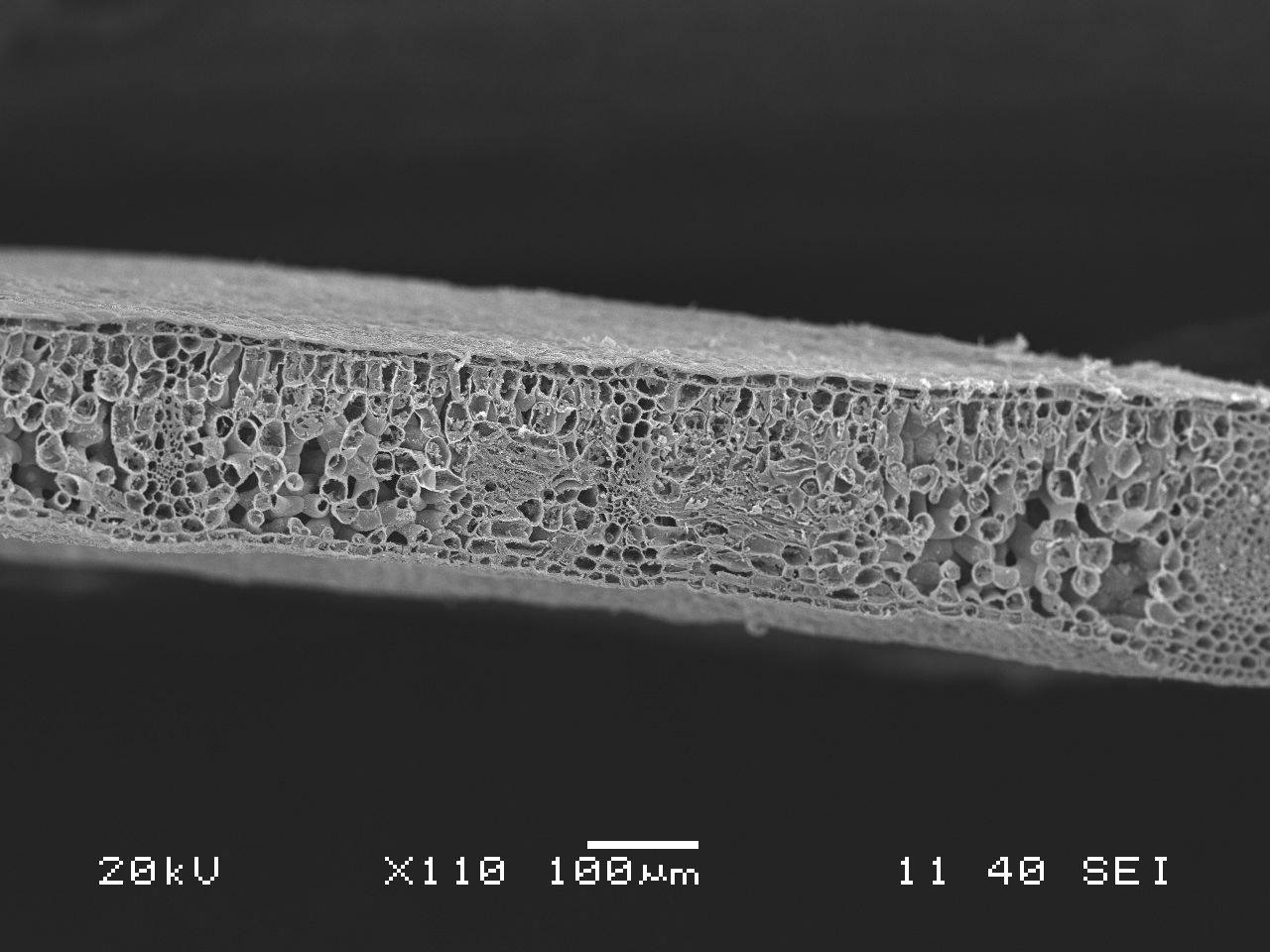
SEM sample prep – freeze fracturing with liquid Nitrogen
The long awaited freeze fracturing with liquid Nitrogen is finally here! We were thrilled to get to do this procedure and to see the results (see post “Freeze fracture samples under the SEM“). A brief overview: we took infected leaves from a madrone, salal and Douglas-fir, fixed them in glutaraldehyde, dried them in ethanol, froze them in liquid nitrogen, smashed them to bits while frozen, dried them further in the critical point drier, stuck them on stubs and finally coated them with gold-palladium in the sputter coater. Wow – Quite a process! It took us the better part of a day (and some of the day before), and we learned through the process.
After the prep process, they are ready to be looked at with the SEM, or Scanning Electron Microscope.
A special thank you to SITs Ladd, Sina and Dan for all the help – and of course, thank you to Dave and Pranav in Dirks’ lab, who gave us the procedure and were patient with all our questions.
Here are our leaf samples in glutaraldehyde. Glutaraldehyde fixes the samples by crosslinking the proteins which kills the cells quickly. As opposed to drying, where cells shrivel up and lose their structure, fixing these leaves in 2% glutaraldehyde stabilized and preserved the cell structure. As you can see, the samples are floating. We wanted them to be fully immersed in the fixative, so we hooked up the bottle to a vacuum system to pull the air out of the same (see below!)
After the leaves sat in fixative overnight, we rinsed them with Millonig’s buffer and soaked them in increasingly more concentrated solutions of ethanol. This was easy, but time consuming.

After the ethanol has frozen, the tray is fully submersed in the liquid Nitrogen for several minutes.

Checking to see if the fracturing worked… it didn’t… so we repeated the freezing process two more times.

Third time is the charm! Note: the samples did not stay separated. This we will have to think about for next time.

Leaf samples, after being fractured and dried, stuck to the grooved SEM studs with conductive paint.
Sputter coating the sample with a thin (THIN) coating of gold-palladium. After this, we’re ready to look at it with the SEM… unfortunately, the process took us so long we had to wait until the next day!
WSU Puyallup Plant Pathology Labs
Today we visited the Plant Pathology Labs at WSU in Puyallup. Jenny Glass, Marianne Elliot and Gary Chastagner gave us a warm welcome.
Jenny Glass, on the left, works for WSU in the Plant & Insect Diagnostic Lab
Here, Yianna looks at a fungus on a potato
Marianne Elliott works in the Plant Pathology Lab at WSU. Her work focuses on Phytophthora species.
This is the thick walled reproductive structure of Phytophthora called a chlamydospore, which can lay dormant in the soil for decades.
Gary Chastagner explains the hardships of the Christmas tree growing business.
A canker on a fir tree
Marianne shows us the leaf blight on a madrone.
Madrone Foliar Pathogens
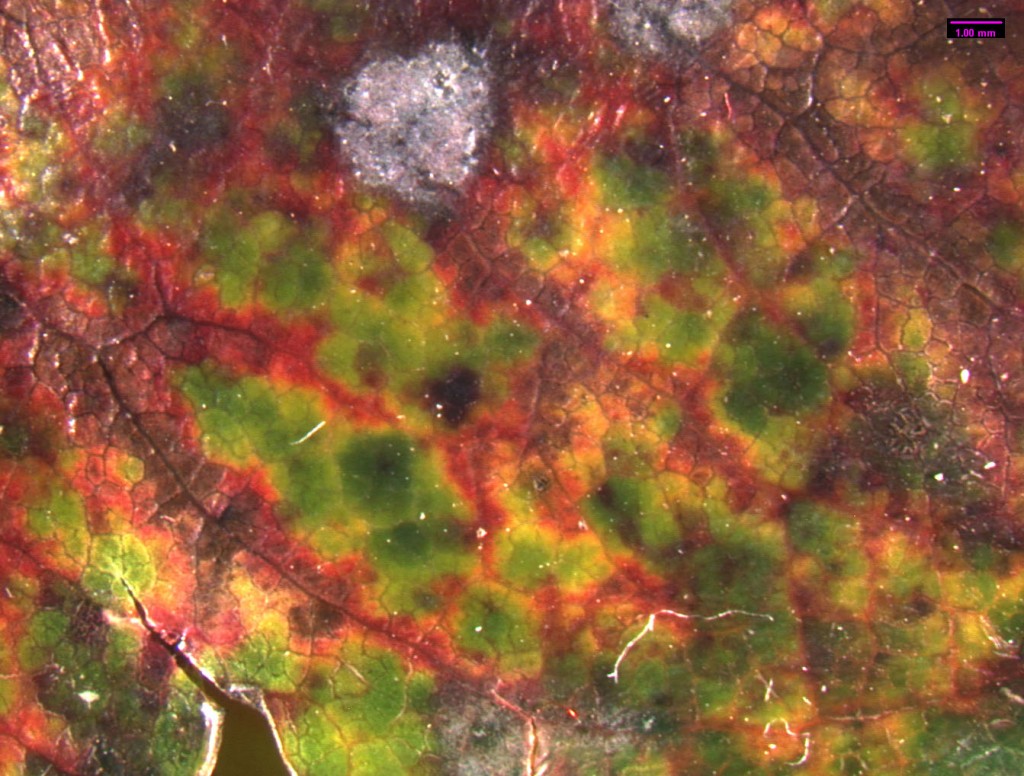 Leaves are prime targets for pathogenic fungi, because they have high concentrations of simple sugars. Leaves generally remain on the plant for around 2 years, then they separate when new leaves are forming in the early spring. Madrone is a broadleaf evergreen, and it has many foliar fungi. Foliar pathogens are more successful at colonizing older, aging leaves than new leaves. In Madrone, new foliage is rarely infected until fall (Hunt and Funk 1992). Spores are produced on infected leaves from the previous year or in lesions on the branches. They disperse through wind and rain. Leaf pathogens consist mostly of Ascomycetes of Deutromycetes. Foliage diseases intensify during periods of warm, wet weather. In some years, there is heavy damage to foliage and most is lost. The infected foliage is replaced by new leaves in summer, and the trees look healthier than others around them that may be suffering from summer drought. Coccomyces quadratus and Rhytisma arbuti attack only madrone (Hunt and Funk 1992). They both reproduce by means of sexual spores that are released when wet. The fruiting body of R. arbuti is a speckled tar spot. Early in the season, the fungus produces pycnidia with single celled asexual spores. The infected tissues later become sclerotia. It also forms apothecia with sexual spores that are long and narrow. This fungus is one of the most damaging as it can overwinter on attached foliage. Coccomyces quadratus forms a large tar spot. Most foliar pathogens (including the sexual stage of Fusicoccum aesculi) are Ascomycetes. They all have a similar life cycle. The sexual spores (ascospores) are released from the fruiting body on the leaf. This usually happens during wet weather, particularly in the spring. The ascospores land on another leaf of a suitable host and germinate. They colonize the leaf and form another fruiting body and asexual spores (conidia). In the summer the conidia are dispersed by wind and rain splash, and infecting more leaves. The mycelium continues to produce conidia as long as conditions are favorable, and large quantities are produced in a season. The same mycelium then produces asci, and genetic maerial is exchanged between mycelia of compatible mating types. The ascospores overwinter on dead leaves and begin the cycle again in the spring.
Leaves are prime targets for pathogenic fungi, because they have high concentrations of simple sugars. Leaves generally remain on the plant for around 2 years, then they separate when new leaves are forming in the early spring. Madrone is a broadleaf evergreen, and it has many foliar fungi. Foliar pathogens are more successful at colonizing older, aging leaves than new leaves. In Madrone, new foliage is rarely infected until fall (Hunt and Funk 1992). Spores are produced on infected leaves from the previous year or in lesions on the branches. They disperse through wind and rain. Leaf pathogens consist mostly of Ascomycetes of Deutromycetes. Foliage diseases intensify during periods of warm, wet weather. In some years, there is heavy damage to foliage and most is lost. The infected foliage is replaced by new leaves in summer, and the trees look healthier than others around them that may be suffering from summer drought. Coccomyces quadratus and Rhytisma arbuti attack only madrone (Hunt and Funk 1992). They both reproduce by means of sexual spores that are released when wet. The fruiting body of R. arbuti is a speckled tar spot. Early in the season, the fungus produces pycnidia with single celled asexual spores. The infected tissues later become sclerotia. It also forms apothecia with sexual spores that are long and narrow. This fungus is one of the most damaging as it can overwinter on attached foliage. Coccomyces quadratus forms a large tar spot. Most foliar pathogens (including the sexual stage of Fusicoccum aesculi) are Ascomycetes. They all have a similar life cycle. The sexual spores (ascospores) are released from the fruiting body on the leaf. This usually happens during wet weather, particularly in the spring. The ascospores land on another leaf of a suitable host and germinate. They colonize the leaf and form another fruiting body and asexual spores (conidia). In the summer the conidia are dispersed by wind and rain splash, and infecting more leaves. The mycelium continues to produce conidia as long as conditions are favorable, and large quantities are produced in a season. The same mycelium then produces asci, and genetic maerial is exchanged between mycelia of compatible mating types. The ascospores overwinter on dead leaves and begin the cycle again in the spring.
Didymosporium arbuticola and Diplogia maculata are asexual fungi found only on madrone. Their fruiting bodies are small leaf spots. Didymosporium arbuticola forms brown spots 3-6 mm in diameter with purple to reddish margins (Hepting 1971). Blister blight (exobasidium vaccinii) forms pinkish blister-like galls that twist and distort leaves. It attacks the fruit, making it turn red and swell to several time its natural size. Basidiospores are formed on the underside of the leaf blisters and on the surface of the infected fruits in a thin layer of fungal tissue. They are ejected during wet weather. Foliage diseases rarely threaten the survival of the tree; however, persistent foliage disease may predispose trees to attack by other fungi. Inoculum can be reduced by removing dead leaves and pruning infected twigs. Unraked leaves are a source of inoculum in the late summer or early fall. Splashing the foliage when watering will spread spores.
Madrone foliar pathogens info found @
http://soilslab.cfr.washington.edu/madrone/ch07_el.pdf
Marianne, Elliot. Diseases of Pacific Madrone.
Citations
Hepting, G.H. 1971. Diseases of Forest and Shade Trees of the UnitedStates. USDA Forest Service. Handbook #386:76–78.
Hunt, R.S, and A. Funk. 1992. Common Pests of Arbutus. Canadian
Forestry Service, Victoria, British Columbia. FPL 63.
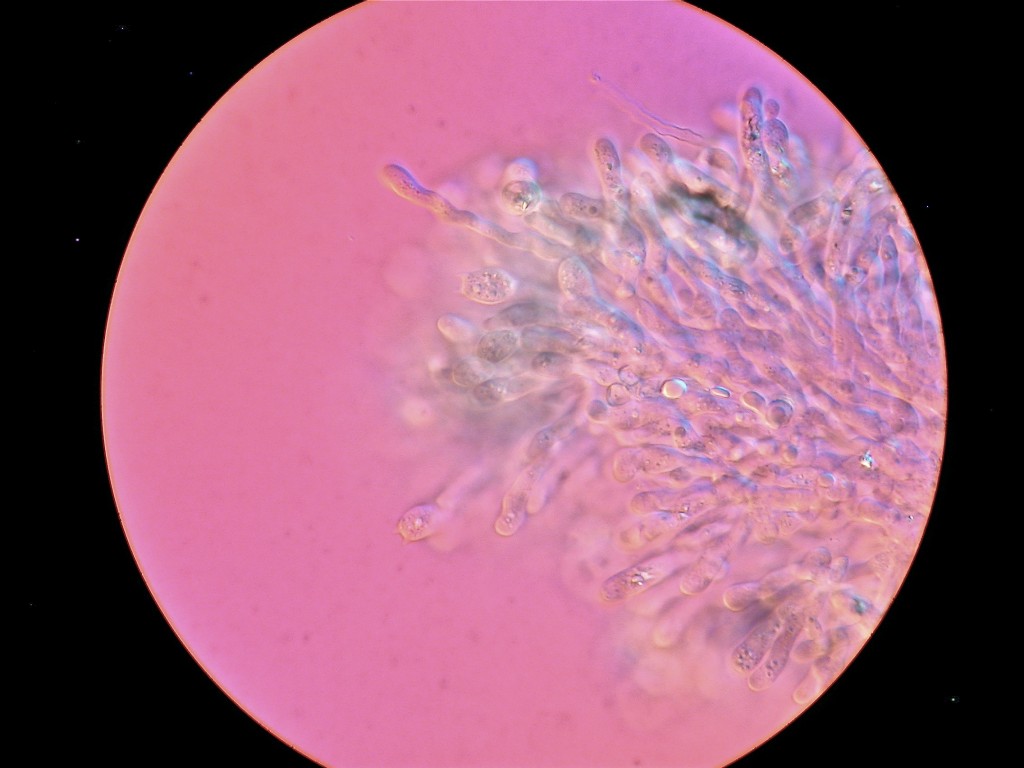
Hericium abietis
Today we looked at Hericium abietis with the Zeiss microscope. This picture, of the tip of one of the teeth from a culture, was taken with the DIC on the microscope.
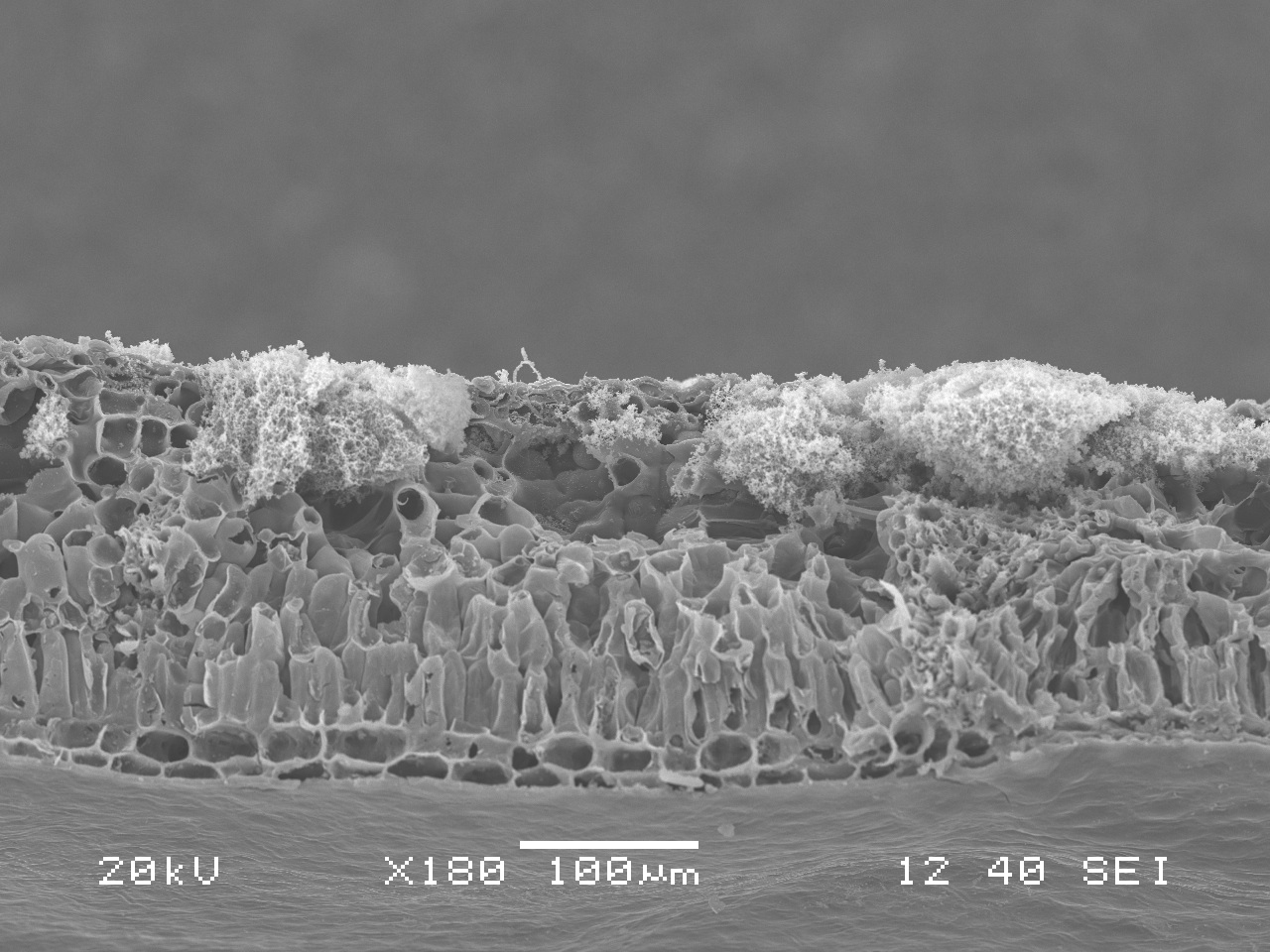
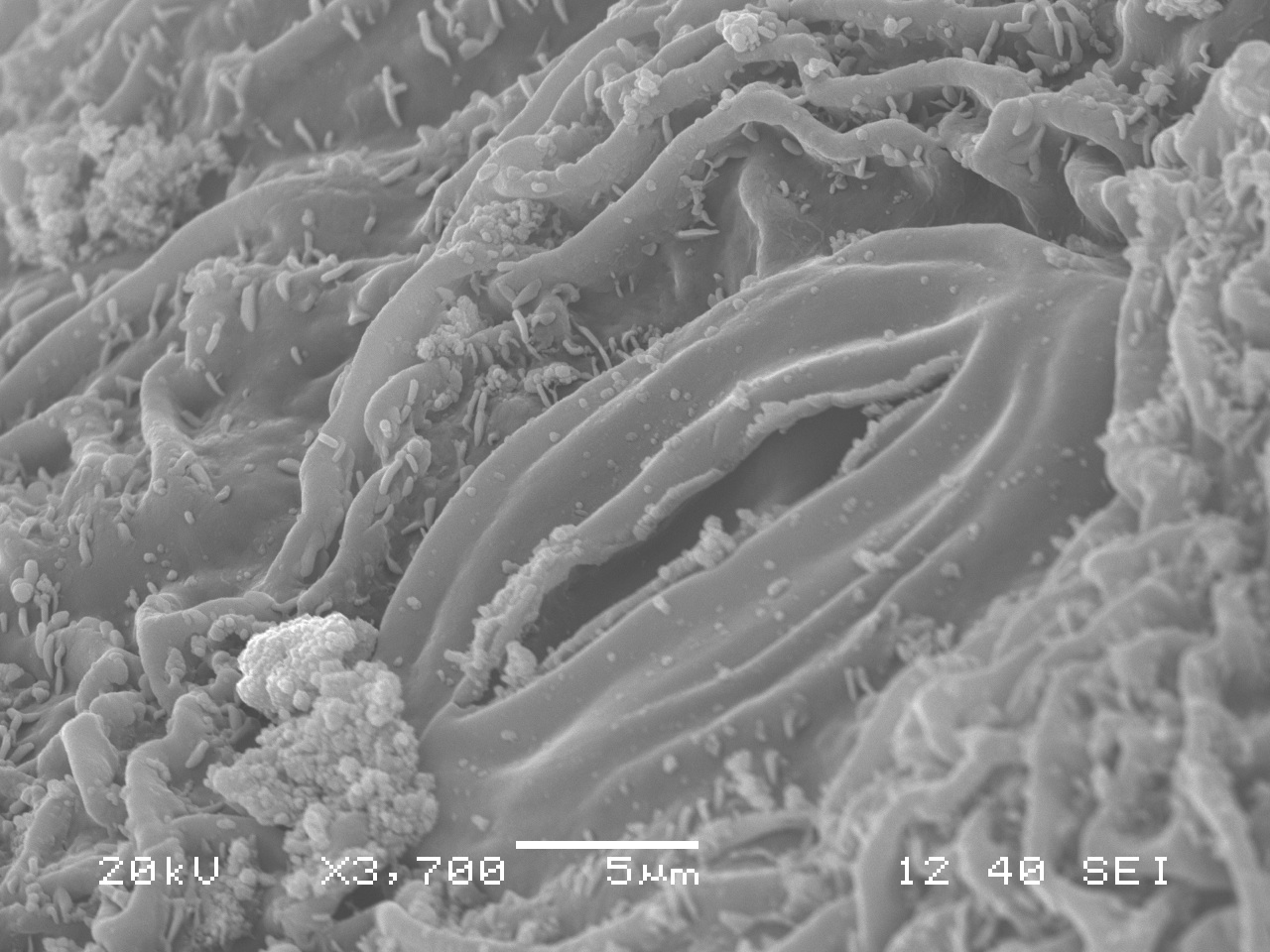
Swiss Needle Cast under the SEM
This is a close-up of a Douglas fir needle infected with Swiss Needle Cast. Here you can clearly see some healthy, unclogged stomata, as well as some stomata clogged by the fungi. You can also see the hyphae in the bottom photo.
Our Most Recent Search In the TESC Forest
Today we found the telltale black rhizomorphs of the pathogenic fungus Armillaria ostoyae growing on a long dead snag, just off of the organic farm trail.

We also found several trees that have fallen due to fungal infection. In a healthy tree there would be a root disk present, with the tree having fallen with most of its roots mostly intact. In the case of these infected trees the roots are damaged by the fungus and they all snapped off, with many of the roots separate from the log and still jutting out of the ground.


Here is a plaque that was dedicated to an opening in the forest canopy, next to the organic farm trail. Its dedicated to a large laminated root rot infection within that part of the forest.
Preparation of a Swiss Needle Cast sample for the SEM
We prepped our Swiss Needle Cast infected Douglas Fir needles today for the SEM. Have a look!
Globalization, Climate Change, & an Increase in Pathogenic Forest Fungi
Globalization means that many human diseases such as H1N1, SARS, ebola and other killer diseases can travel swiftly with ease around the globe, infecting new human populations. However, the rapid pace of globalization not only affects us humans, but impacts forest ecosystems the world over as well. The quick passage of people and goods across the globe has also dramatically increased the mobility of fungi, parasites, and viruses. Hitching rides like vagabond bums, they cling to the agricultural produce in freight trains, hide within nursery stock in the back of landscaping trucks and sleep in timber in the back of logging trucks. They’ll catch a ride with any unsuspecting traveler who unknowingly picks them up on the bottom of their shoes, clothing, or in tools that the timber industry uses in the field. Goods that are being traded all over the world are smuggling pathogens along with them all across their trade routes! Globalization is challenging native species with unique and exotic species combinations that have never before existed, and unfortunately in this species vs. species battle, pathogens seem to have the upper-hand over the trees every time. Fungi are able to grow swiftly, and reproduce multiple generations within a matter of weeks whereas trees by contrast take years to reach maturity, let alone reproduce, which severely curtails their ability to adapt to new stresses from exotic pathogens they’ve never had to deal with before. In addition, native pathogens that are already prevalent may have the upper-hand in scenarios of increased stress, such as long-term climate change.
Climate change also plays a significant role in this fight, usually favoring the fungi and other pathogens. There is evidence that increased warming enhances pathogen fruiting periods. The accelerating climate changes, coupled with stressful monocrop style forestry practices, puts considerable strain on trees which then have a much higher susceptibility to pathogen attacks. Shifts in a forests climate, precipitation, pH levels, etc. can alter tree physiology and compromise the trees natural resistance to pathogenic invaders. This is complicated by the decline of our forests, which are being swallowed by modern development. This effectively makes the forest an island at sea, with its tiny shores being easily pillaged by newcomers, delighting in the spoils of an unsuspecting ecosystem. This threat of colonization of our forests by invasive species is not restricted to pathogenic fungi but also plants and insects that can associate with fungal pathogens, acting as the main source where the plague can spread from.
This brutal cocktail of a rapidly changing climate mixed with teeming multitudes of advantageous fungi means that many Northwestern forests will have to face dramatic shifts in species compositions in the coming decades. Larger, older hardwoods can be expected to get pushed out by shrubs and more well adapted, less vulnerable species of trees. For now, it seems, the best chance that trees have is the increased human interest and research into fungi and pathogens by scientists committed to understanding the various diseases that affect our forests.
Swiss Needle Cast
We found Phaeocryptus gaumanni, Swiss Needle Cast, in the forest at Evergreen today. The host is Pseudotsuga menziesii (Douglas-fir).
The black pseudothecia block the stomates in the needles which prevents respiration.
Priest Point Pathogens
We met up with Tom Otto and Ira Davis, arborists for the City of Olympia, and they showed us the pathogens in Priest Point Park. Here are some pictures of our experience:
Tom ousting the middle of the remains of a Phaeolus schweinitzii casualty. This tree was a Douglas fir when it was still alive, the cubicle rot of the wood is characteristic of the rot caused by this species.
This big leaf maple had to be cut because of a rot occurring at the base. The neighboring maple had a similar rot and fell in the middle of the parking lot; luckily no one was hurt! They cut this tree down before the Hypoxilon rotted it enough to completely fall over at an unpredictable time.
Here is a picture of the culprit, Hypoxilon.
Here is a great example of Phellinus sulfarescens (formely P. wereii). Isaac cut the rot out, and we will curate this into our pathogen teaching collection.
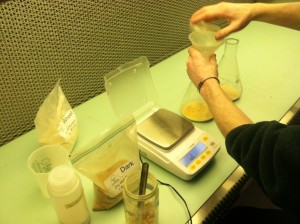
Pouring plates!
We poured our first agar plates today! Thanks to Zach for helping us and giving us the recipe.
Here is the recipe if anyone is curious:
- 1000 ml DI water
- 20g agar
- 20g malt
- 2g yeast