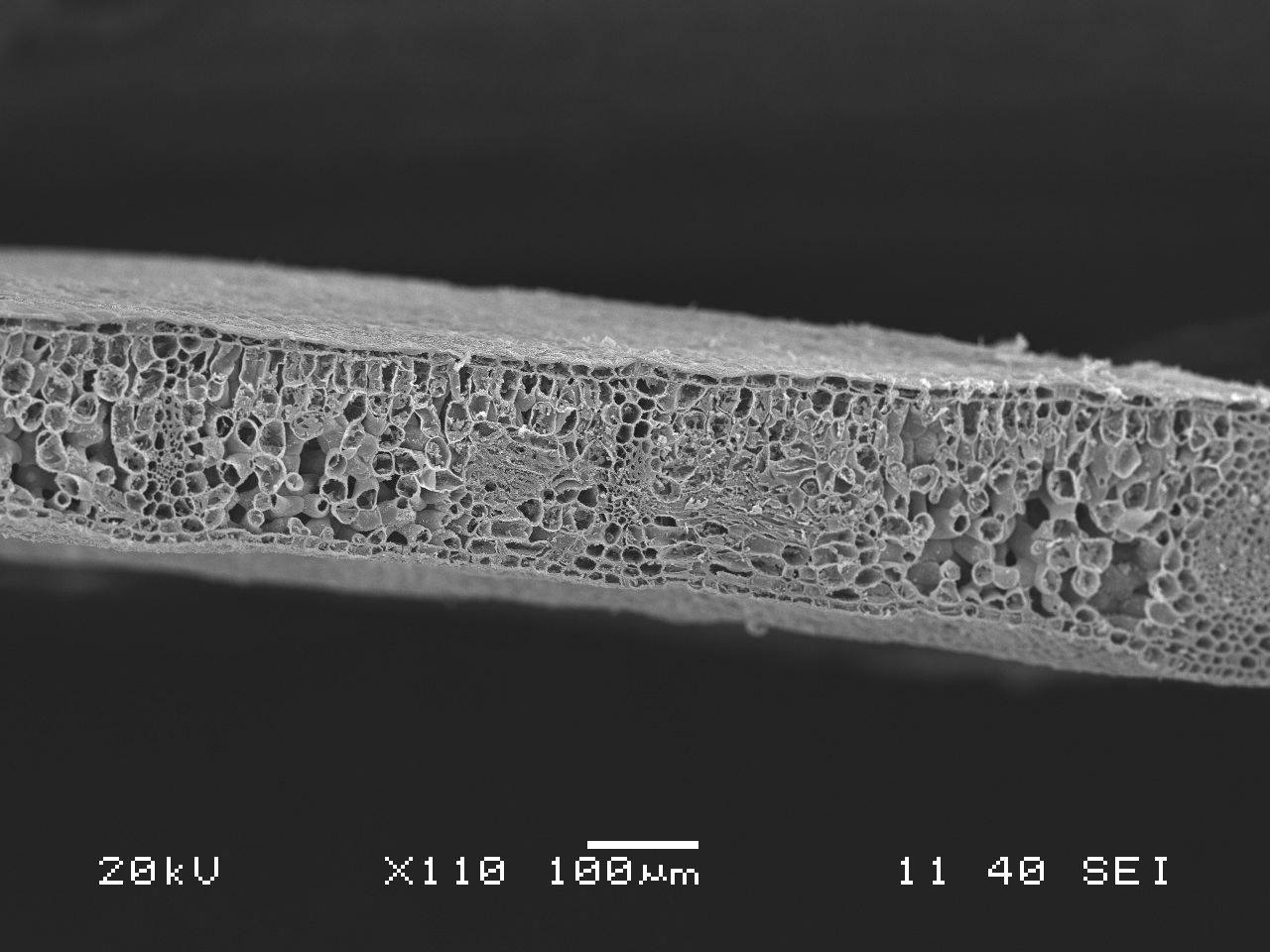
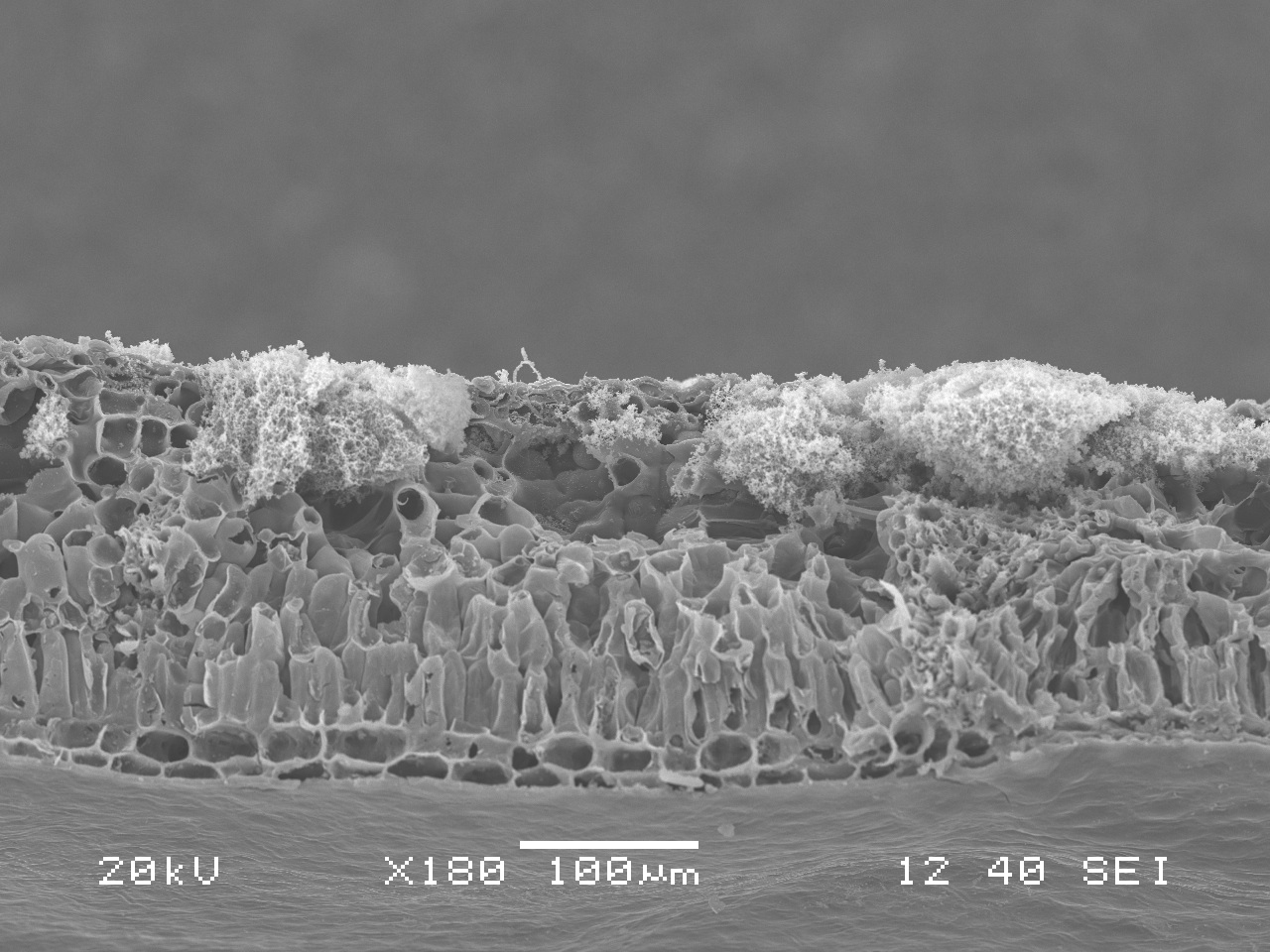
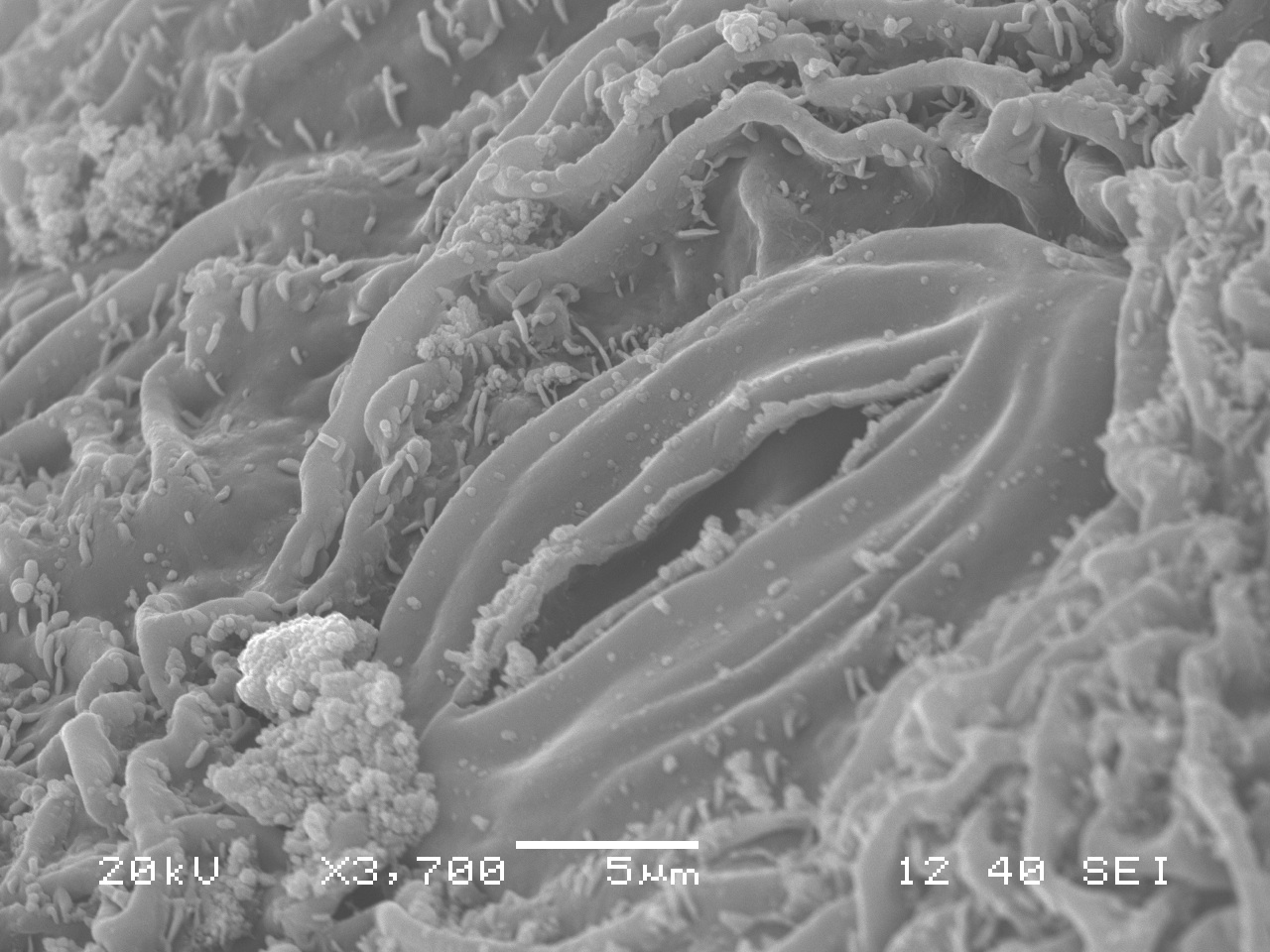
Are pathogenic fungi lurking in our Washington forests and if so, what are their impacts? What deadly creatures conceal themselves in the soil depths and creep beneath the roots of the trees we see every day? Thusly we began our quest to find these unknown terrors!
Let’s take a walk through the forest and see what ghastly rots may be in store. Imagine we are walking through the woods in western Washington. Just this once, it’s not raining. We start on the path at an ambling pace, with an eye out for anything interesting. What do you see? There are trees, shrubs, and the path in front of you. There is moss everywhere. To your left you can see a leaning snag sporting several hefty shelf mushrooms. To your right there is a sad looking western hemlock surrounded by the pervasive honey mushroom, Armillaria. The honey mushroom is a pathogen, and will eat its host to death while its root-like rhizomorphs creep toward neighboring trees.
A pathogen is an organism that causes disease in another living organism. The organism the pathogen parasitizes is called the host. Most types of living creatures including plants and animals can be host to pathogens. Some pathogens of forest plants form mushrooms, one of the more conspicuous signs of infection. Mushrooms, also known as fruiting bodies, are reproductive structures of fungi analogous to the fruit of an apple tree. Fruiting bodies are one way that fungi produce spores, reproductive structures that can drift through air or water, or just lay wait in the soil, eventually landing on and infecting a new susceptible host. Fungi can grow in soil and spread to roots, bark, and heartwood of trees, and not all produce fruiting bodies. For this reason, you might only be able to see symptoms of disease, such as the unhealthy yellowing of needles on this Douglas-fir beside the trail, or a healthy looking tree leaning in the midst of a canopy gap in the woods.
Our research on pathogenic fungi causes us to seek out these dejected trees. Our group works to collect, to preserve, to teach. We have sought information from pathogen experts throughout the area, who gladly helped us in our project to create a teaching collection for future students. We have been enthusiastically gathering mushrooms sprouting from trunks, yellowing needles with plugged stomata, hole-ridden roots and laminated annular rings. We poked, prepared, processed, took pictures, personified, pondered and persisted in our project. It soon became clear to see how important pathogen disease is in keeping the ecosystem of our forests balanced.
Fungi are able to grow swiftly, and can sometimes reproduce multiple generations within a matter of weeks. In contrast, trees take years to reach maturity, let alone reproduce, resulting in a timeline which severely curtails their ability to adapt to new stresses. Recent studies have predicted an increase in pathogen fruiting periods in response to warming, which will strain host species (Roy et al 2004 and Shafer et al 2001). An accelerating climate change coupled with monocrop-style forestry practices puts considerable stress on trees, which are then more susceptible to pathogen infection. Matters are further complicated by the disappearance of healthy forests as they are swallowed up by modern development. This combination of unprecedented climate change, industrialization, and advantageous pathogenic fungi creates the potential for a dramatic shift in Northwest forests.
In spite of all this doom and gloom, plants are typically equipped with the means to fend off hungry pathogens. Their cell walls are made of cellulose and lignin, which are tough protective materials. Plants synthesize antimicrobial secondary metabolites to battle threatening invaders and produce detoxifying enzymes to break down the compounds that pathogens use to weaken plant tissues. When sensing a fungal invasion, some plants develop a thick structure inside the cell to wall off the offending fungus and protect the cell. A plant will even kill off its own infected (and nearby healthy) cells in what is called a hypersensitive response. This prevents further infection and can be a small sacrifice for the overall health of the plant. Alas, despite these defensive efforts, many plants still yield to the relentless pathogenic pressure.

Swiss Needle Cast
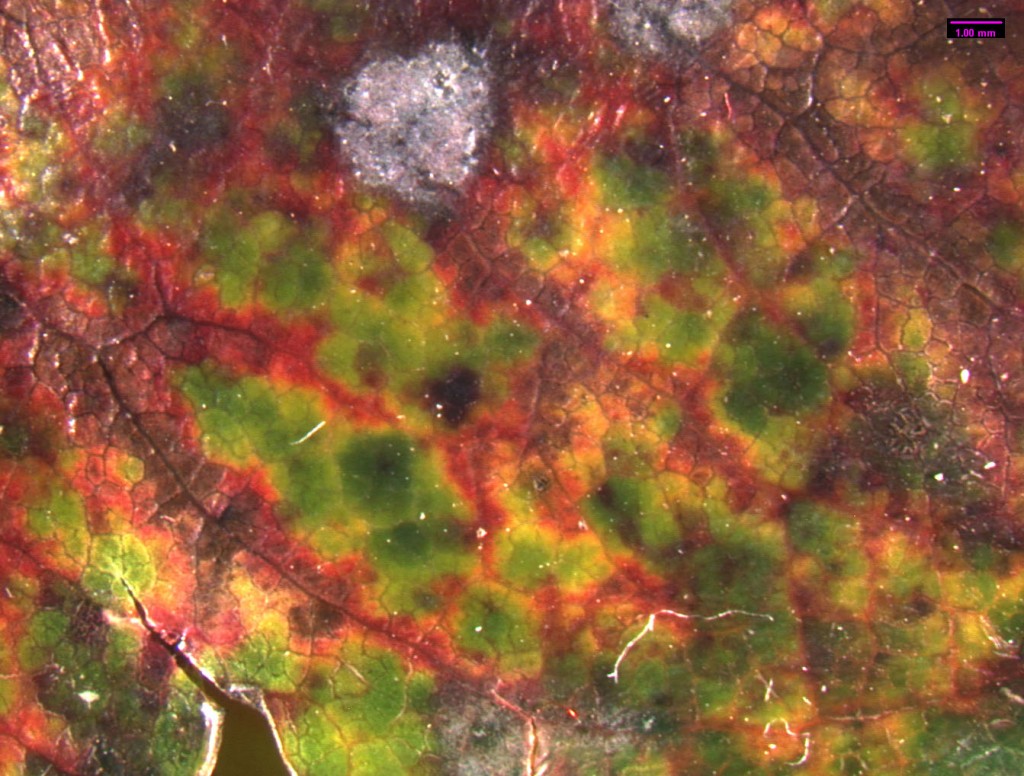
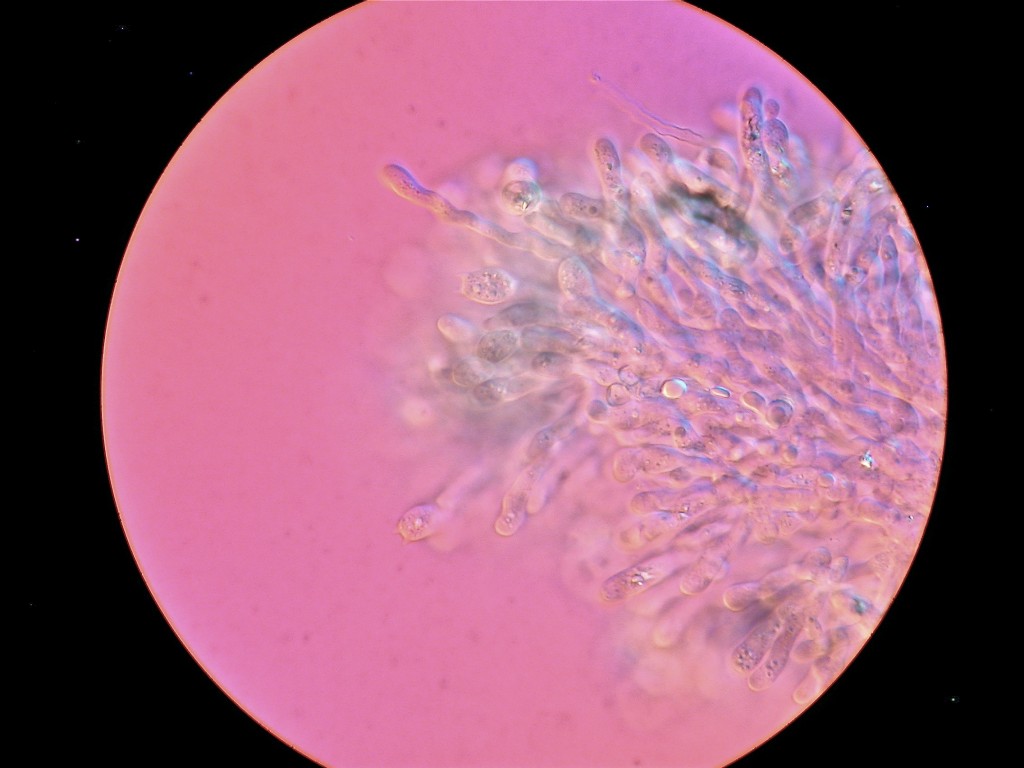
Standing under the sad-looking western hemlock, you think a little bit more about the honey mushrooms consuming it. What will be the fate of this tree? What other pathogens grow in these woods? You notice the corky bark of a Douglas-fir. Its bark looks healthy, but the needles are turning a sickly yellow. Pull out your hand lens, you may be able to see the tiny black fruiting bodies of the culprit: Swiss Needle Cast (Phaeocryptopus gaeumannii) (Figure 1). This is one of the most common foliar pathogens in our area. Swiss Needle Cast’s damage is not just cosmetic. The fungus plugs up the stomata, small openings in the needles that act like little noses for the tree, and the tree can’t breathe well enough to photosynthesize properly.
You notice the tree next door has toppled over. Walk over and take a closer look at the decay. You see the roots still buried in the ground with the rest of the trunk cracked and broken off a few feet from the forest floor. Check inside the stump. You’ll see the inner layers of wood have “delaminated” and peeled apart to form separated layers like thick pages of a book. This is the work of Washington’s number one tree killer, Laminated Root Rot (Phellinus sulphurascens).
In the end, we began to realize fungal pathogens are actually a vital part of a healthy forest ecosystem. We needn’t be so harsh with our adjectives and peg creepy, sneaky, invading pathogenic fungi as a plague upon the forest. The truth is, they also enhance forest ecosystems by creating canopy gaps and increasing availability of nutrients to other organisms (Hansen et al 2000, Laine 2004). Our sad-looking western hemlock will eventually fall, but the honey mushroom will continue to feed on the tree and recycle the nutrients back into the forest. The gap left in the canopy will let sunlight reach understory vegetation, which will give rise to other species that had been competing for light. This hemlock is not alone in its fate. Death by fungi isn’t always pretty, but it has an important role in the forest. Through our research we realized that the plague begins when an altered environment allows pathogens to grow out of control and throw an ecosystem out of balance. Next time you set off down that open trail keep in mind the delicate balance of give and take in nature around you.
Sources:
Hansen, EM; Goheen, EM. 2000. Phellinus weirii and Other Native Root Pathogens As Determinants of Forest Structure and Process in Western North America. Annual Review of Phytopathology, 38: 515-39.
Laine, A. 2004. Resistance Variation within and among Host Populations in a Plant: Pathogen Metapopulation: Implications for Regional Pathogen Dynamics. Journal of Ecology, 92: 990-1000
Roy, B; Güsewell, S and Harte J. 2004. Potential Changes in the Distributions of
Western North America Tree and Shrub Taxa under Future Climate Scenarios. Ecology,
Vol. 85, No. 9: 2570-2581
Shafer, SL; Bartlein, PJ and Thompson, RS. 2001. Potential Changes in the Distributions of Western North America Tree and Shrub Taxa under Future Climate Scenarios. Ecosystems, Vol. 4: 200-215